Liquid ventilator
A liquid ventilator is similar to a medical ventilator except that it should be able to ensure reliable total liquid ventilation with a breatheable liquid (a perfluorocarbon)[1] · .[2] Liquid ventilators are prototypes that may have been used for animal experimentations but experts recommend continued development of a liquid ventilator toward clinical applications.[3]

Function and technology
Driving liquid
In total liquid ventilation (TLV), the lungs are completely filled with a perfluorocarbon (PFC) liquid while the liquid ventilator renews the tidal volume of PFC. The liquid ventilator operates in mandatory mode: it must force the PFC in and out of the lungs with a pumping system.
- During the inspiratory phase, the pump generates a positive driving pressure in the trachea to ensure the PFC insertion of the tidal volume.
- During the expiratory phase, the pump generates a negative driving pressure in the trachea to ensure PFC withdrawal of the tidal volume.
The pumping system is either a peristaltic pump (in the simplest liquid ventilators) or two piston pumps (in the most advanced liquid ventilators).
Because of the PFC viscosity, the head loss in the airways requires a low negative driving pressure during the expiration phase that can collapse the airways. This is the choked flow phenomenon in TLV[4] · [5] which compromises the minute ventilation and consequently the gas exchanges.[6] To address this limitation, liquid ventilator integrates a control of the pumping system.
Controlling liquid ventilator
The introduction of computers in liquid ventilators to control the pumping system provides different control modes, monitoring and valuable data for decision making[7] · .[8]
The liquid ventilator is always volume-controlled because the specified tidal volume of PFC must be accurately delivered and retrieved. It is also pressure-limited because it must stop the expiratory or inspiratory phase when a too low, or a too large, driving pressure is detected.[9]
However, during the expiratory phase, the expiratory flow can be commanded by an open-loop controller or a closed-loop controller:
- when the expiratory flow is open-loop controlled, it is fast initially and slowing down progressively after to minimize the risk of collapse generation.[10] · [11]
- when the expiratory flow is closed-loop controlled, it is commanded in real-time to maintain a specified driving pressure. This is a pressure-regulated mode. Such approach automatically avoids collapse generation.[12]
Also, during the inspiratory phase, the volume-controlled mode is realized by open-loop or closed loop control of the PFC flow.
Oxygenating and heating liquid
The liquid ventilator removes Carbon dioxide (CO2) from the PFC by saturating it with oxygen (O2) and medical air. This procedure can be performed with either a membrane oxygenator (a technology used in extracorporeal oxygenators) or a bubble oxygenator.[13]
The liquid ventilator heats the PFC to body temperature. This is performed with a heat exchanger connected to the oxygenator or with dedicated heaters integrated in the oxygenator.[13]
The oxygenator and the heater produce PFC vapor which is recuperated with a condenser in order to limit the evaporation loss (the PFC is a greenhouse gas).
Example
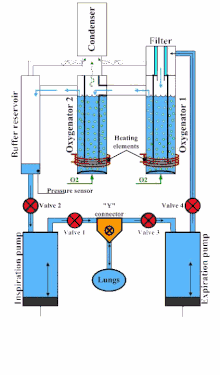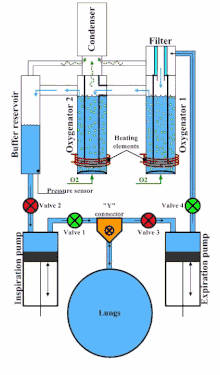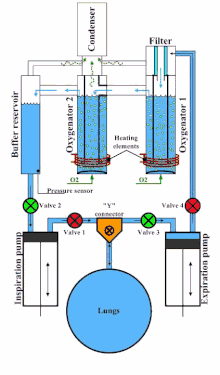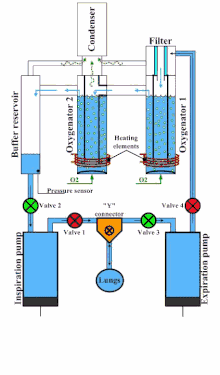
An example of a liquid ventilator is the Inolivent-4. It is composed of two independent piston pumps and integrated unit allowing for oxygenation of PFC, temperature control, and recovery of evaporated PFC.[13] This liquid ventilator also includes volume and pressure control strategies to optimize the ventilatory cycle: it performs a pressure-regulated volume-controlled ventilation mode.[12] It is designed for experimental research on animal models weighing between 0.5 kg to 9 kg.
A typical cycle is composed of four steps :
- Inspiratory pump inserts a volume of PFC in the lungs (valve 1 open, valve 2 closed), and the expiratory pump pushes PFC in the oxygenator via the filter (valve 3 closed, valve 4 open).
- During the inspiratory pause (all valves are closed), the lung volume is at its maximal value. The measured pressure is the Positive End-Inspiratory Pressure (PEIP).
- Expiratory pump retrieves a volume of PFC in the lungs (valve 3 open, valve 4 closed), and the inspiratory pump draws PFC from the reservoir (valve 1 closed, valve 2 open).
- During the expiratory pause (all valves are closed), the lung volume is at its minimal value. The measured pressure is the Positive End-expiratory Pressure (PEEP).
Potential applications
Studies have shown both the efficacy and safety of liquid ventilation in normal, mature and immature newborn lungs. Overall, liquid ventilation improves gas exchange and lung compliance and prevents the lungs against ventilation-induced lung injury.[1]
Respiratory support
Studies suggest clear benefits of liquid ventilation in acute respiratory distress syndrome (ARDS).[14] For example, total liquid ventilation could be used for newborns with severe neonatal respiratory distress syndrome[15] in which conventional treatment has failed. Typical cases are late preterm newborns who have an increased risk of intracranial hemorrhage and for whom their small vessel size poses technical limitations for Extracorporeal membrane oxygenation (ECMO).
Therapeutic lung lavage
Liquid ventilator can perform therapeutic lung lavage, the washout of endogenous and exogenous debris from the lungs, without suspension of ventilation support (without apnea). For example, literature data suggest a radical change in the treatment of meconium aspiration syndrome (MAS) by considering the use of a liquid ventilator. The demonstration of its efficacy was performed in the neonatal lamb.[16] · .[17]
Therapeutic hypothermia with rapid cooling
The liquid ventilator with advanced control temperature of PFC allows the rapid cooling of the body. Consequently, therapeutic hypothermia is an expected clinical application. For example, studies present that rapid cooling instituted by TLV can improve cardiac and mitochondrial function [18] or can induce favorable neurological and cardiac outcomes after cardiac arrest in rabbits.[19]
See also
References
- M. R. Wolfson; T. H. Shaffer (2005). "Pulmonary applications of perfluorochemical liquids: ventilation and beyond". Paediatr Respir Rev. 6 (2): 117–27. doi:10.1016/j.prrv.2005.03.010. PMID 15911457.
- Kaisers, K., Kelly, K.P., Busch, T. (2003). "Liquid ventilation". British Journal of Anaesthesia. 91 (1): 143–151. doi:10.1093/bja/aeg147. PMID 12821573.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Maria Laura Costantino; Philippe Micheau; Thomas H. Shaffer; Stefano Tredici; Maria R. Wolfson (2009). "Clinical Design Functions: Round table discussions on bioengineering of liquid ventilators". ASAIO J. 55 (3): 206–8. doi:10.1097/MAT.0b013e318199c167. PMID 19282746.
- Baba; Brant, D; Brah, SS; Grotberg, J; Bartlett, RH; Hirschl, RB; et al. (2004). "Assessment of the development of choked flow during liquid ventilation". Crit. Care Med. 32 (1): 201–208. doi:10.1097/01.CCM.0000104918.48411.91. PMID 14707580.
- Bull; Foley, DS; Bagnoli, P; Tredici, S; Brant, DO; Hirschl, RB; et al. (2005). "Location of Flow Limitation in Liquid Filled Rabbit Lungs". ASAIO J. 51 (6): 781–788. doi:10.1097/01.mat.0000179252.02471.9e. PMID 16340368.
- D. Corno; G.B. Fiore; M.L. Costantino (2004). "A mathematical model of neonatal tidal liquid ventilation integrating airway mechanics and gas transfer phenomena". IEEE Trans. Biomed. Eng. 51 (4): 604–611. doi:10.1109/TBME.2004.824144. PMID 15072214.
- Sekins; Nugent, L; Mazzoni, M; Flanagan, C; Neer, L; Rozenberg, A; Hoffman, J; et al. (1999). "Recent innovation in total liquid ventilation system and component design". Biomed. Eng. & Tech. 33 (3): 277–284. PMID 10360218.
- JL. Heckman; J Hoffman; TH Shaffer; MR Wolfson (1999). "Software for real-time control of a tidal liquid ventilator". Biomedical Instrumentation & Technology. 33 (3): 268–276.
- Larrabe; et al. (October 2001). "Development of a time-cycled volume-controlled pressure-limited respirator and lung mechanics system for total liquid ventilation". IEEE Trans Biomed Eng. 48 (10): 1134–44. doi:10.1109/10.951516. PMID 11585037.
- R. Robert; P. Micheau; O. Avoine; B. Beaudry; H. Walti (2009). "A Regulator for Pressure Controlled Liquid Ventilation". IEEE Trans. Biomed. Eng. 57 (9): 2267–76. doi:10.1109/TBME.2009.2031096. PMID 19744909.
- Tredici; Komori, E; Funakubo, A; Brant, DO; Bull, JL; Bartlett, RH; Hirschl, RB (2004). "A prototype of a liquid ventilator using a novel hollow-fiber oxygenator in a rabbit model". Crit. Care Med. 32 (10): 2104–2109. doi:10.1097/01.CCM.0000142701.41679.1B. PMID 15483421.
- R. Robert; P. Micheau; H. Walti (2009). "Optimal expiratory volume profile in tidal liquid ventilation under steady state conditions, based on a symmetrical lung model". ASAIO J. 55 (1): 63–72. doi:10.1097/MAT.0b013e3181911821. PMID 19092655.
- R. Robert; P. Micheau; S. Cyr; O. Lesur; J.P. Praud; H. Wallti (2005). "A prototype of volume-controlled tidal liquid ventilator using independent piston pumps". ASAIO J. 52 (6): 638–645. doi:10.1097/01.mat.0000249016.31910.11. PMID 17117053.
- Wolfson, M. R., R. B. Hirschl; et al. (2008). "Multicenter comparative study of conventional mechanical gas ventilation to tidal liquid ventilation in oleic acid injured sheep". ASAIO J. 54 (3): 256–269. doi:10.1097/MAT.0b013e318168fef0. PMID 18496275.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hirschl; Pranikoff, T; Gauger, P; Schreiner, RJ; Dechert, R; Bartlett, RH; et al. (1995). "Liquid ventilatory in adults, children, and full-term neonates". Lancet. 346 (8984): 1201–1202. doi:10.1016/S0140-6736(95)92903-7. PMID 7475663.
- R. Foust; N. Tran; et al. (1996). "Liquid assisted ventilation: an alternative ventilatory strategy for acute meconium aspiration injury". Pediatr. Pulmonol. 21 (5): 316–22. doi:10.1002/(SICI)1099-0496(199605)21:5<316::AID-PPUL7>3.0.CO;2-K. PMID 8726157. Archived from the original on 2012-10-19.
- Avoine; et al. (2011). "Total Liquid Ventilation efficacy in an Ovine Model of severe meconium aspiration syndrome". Critical Care Medicine. 39 (5): 1097–103. doi:10.1097/ccm.0b013e31820ead1a. PMID 21317652.
- R. Tissier; N. Couvreur; B. Ghaleh (2009). "Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction". Cardiovasc. Res. 83 (2): 345–53. doi:10.1093/cvr/cvp046. PMC 2701717. PMID 19196828.
- Chenoune; et al. (2011). "Ultrafast and whole-body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits". Circulation. 124 (8): 9011–11. doi:10.1161/circulationaha.111.039388. PMC 3375236. PMID 21810660.