Maitansine
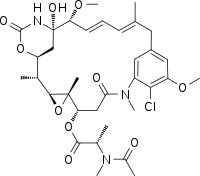
Maitansine (INN), or maytansine (USAN), is a cytotoxic agent. It inhibits the assembly of microtubules by binding to tubulin at the rhizoxin binding site.[1]
 | |
| Names | |
|---|---|
| Other names
Maytansin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.047.944 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C34H46ClN3O10 |
| Molar mass | 692.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is a macrolide of the ansamycin type and can be isolated from plants of the genus Maytenus.[1]
Maytansinoids
Derivatives of maitansine are known as maytansinoids.[2][3] Some are being investigated as the cytotoxic component of antibody-drug conjugates for cancer treatment,[4] and the antibody-drug conjugate trastuzumab emtansine is an approved drug for the treatment of certain kinds of breast cancer in the EU and in the US.[5][6]
Examples of maytansinoids are:
- Ansamitocin[2]
- Mertansine / emtansine (DM1)
- Ravtansine / soravtansine (DM4)
See also
- ImmunoGen, developer of maytansinoid based drugs
References
- National Cancer Institute: Definition of Maytansine
- Yu, T.-W.; Bai, L; Clade, D; Hoffmann, D; Toelzer, S; Trinh, KQ; Xu, J; Moss, SJ; Leistner, E (2002). "The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnemapretiosum". Proceedings of the National Academy of Sciences. 99 (12): 7968–7973. Bibcode:2002PNAS...99.7968Y. doi:10.1073/pnas.092697199. PMC 123004. PMID 12060743.
- Lopus, M; Oroudjev, E; Wilson, L; Wilhelm, S; Widdison, W; Chari, R; Jordan, MA (2010). "Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules". Mol Cancer Ther. 9 (10): 2689–99. doi:10.1158/1535-7163.MCT-10-0644. PMC 2954514. PMID 20937594.
- Chari, RV; Martell, BA; Gross, JL; et al. (January 1992). "Immunoconjugates containing novel maytansinoids: promising anticancer drugs" (PDF). Cancer Res. 52 (1): 127–31. PMID 1727373.
- "Kadcyla EPAR". European Medicines Agency (EMA). 17 September 2018.
- "Drug Approval Package: ado-trastuzumab emtansine". U.S. Food and Drug Administration (FDA). 22 February 2013. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.