Medroxalol
Medroxalol is a vasodilator beta blocker also classified as a mixed receptor blocker as it blocks both alpha and beta receptors.[1]
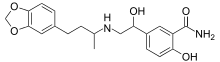 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.618 |
| Chemical and physical data | |
| Formula | C20H24N2O5 |
| Molar mass | 372.421 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
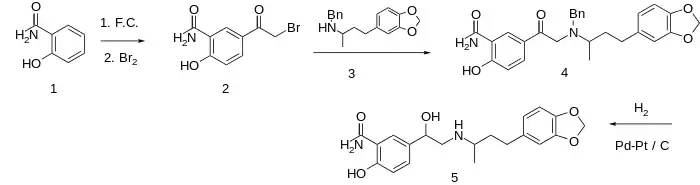
Medroxalol synthesis:[2]
For the first step, salicylamide (1) is the subject of a Friedel-Crafts acetylation and then the aromatic methylketone is halogenated. in the usual manner. The bromide in 2 is then displaced by the nitrogen in N-benzyl-1-(3',4'-methylenedioxyphenyl)-3-butylamine (3), which is itself prepared by reductive amination on the corresponding ketone. The product of the last step (4) is catalytically hydrogenated. This serves the dual purpose both of reducing the ketone and removing the benzyl protecting group affording the product medroxalol (5). Note that a benzyl protecting group is not necessarily used.
See also
References
- Dage RC, Cheng HC, Woodward JK (1981). "Cardiovascular properties of medroxalol, a new antihypertensive drug". Journal of Cardiovascular Pharmacology. 3 (2): 299–315. doi:10.1097/00005344-198103000-00009. PMID 6166802.
- J. T. Suh and T. M. Bare, U.S. Patent 3,883,560; Chem.Abstr. 83, 78914J (1975).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.