Mitapivat
Mitapivat, sold under the brand name Pyrukynd, is a medication used to treat hemolytic anemia.[1] It is taken as the sulfate hydrate salt by mouth.[1] Mitapivat is a pyruvate kinase activator.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Pyrukynd |
| Other names | AG-348, Mitapivat sulfate (USAN US) |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| Chemical and physical data | |
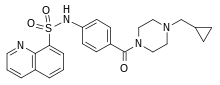
| Formula | C24H26N4O3S |
| Molar mass | 450.56 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mitapivat was approved for medical use in the United States in February 2022.[1][2][3]
Medical uses
Mitapivat is indicated for the treatment of hemolytic anemia in adults with pyruvate kinase deficiency.[1][3]
Pharmacology
Mechanism of action
Mitapivat binds to and activates pyruvate kinase, thereby enhancing glycolytic pathway activity, improving adenosine triphosphate (ATP) levels and reducing 2,3-diphosphoglycerate (2,3-DPG) levels.[4] Mutations in pyruvate kinase cause deficiency in pyruvate kinase which prevents adequate red blood cell (RBC) glycolysis, leading to a buildup of the upstream glycolytic intermediate 2,3-DPG and deficiency in the pyruvate kinase product ATP.[4][5]
Society and culture
Legal status
On 15 September 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Pyrukynd, intended for the treatment of an inherited condition called pyruvate kinase deficiency.[6] The applicant for this medicinal product is Agios Netherlands B.V.[6]
Names
Mitapivat is the international nonproprietary name (INN).[7]
References
- "Pyrukynd- mitapivat tablet, film coated Pyrukynd- mitapivat kit". DailyMed. 23 February 2022. Archived from the original on 3 March 2022. Retrieved 3 March 2022.
- "Agios Announces FDA Approval of Pyrukynd (mitapivat) as First Disease-Modifying Therapy for Hemolytic Anemia in Adults with Pyruvate Kinase Deficiency" (Press release). Agios Pharmaceuticals. 17 February 2022. Archived from the original on 20 February 2022. Retrieved 19 February 2022 – via GlobeNewswire.
- Gormley N. "Pyrukynd (mitapivat) tablets NDA approval" (PDF). Center for Drug Evaluation and Research. Letter to Christina Baladi (Agios Pharmaceuticals, Inc.). U.S. Food and Drug Administration.
- "Mitapivat (Code C157039)". NCI Thesaurus. 31 January 2022. Archived from the original on 20 February 2022. Retrieved 19 February 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "PK-R allosteric activator AG-348". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 10 August 2019. Retrieved 19 February 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Pyrukynd: Pending EC decision". European Medicines Agency (EMA). 15 September 2022. Archived from the original on 19 September 2022. Retrieved 18 September 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3): 539. hdl:10665/330961.
Further reading
- Kung C, Hixon J, Kosinski PA, Cianchetta G, Histen G, Chen Y, et al. (September 2017). "AG-348 enhances pyruvate kinase activity in red blood cells from patients with pyruvate kinase deficiency". Blood. 130 (11): 1347–1356. doi:10.1182/blood-2016-11-753525. PMC 5609468. PMID 28760888.
- Rab MA, Van Oirschot BA, Kosinski PA, Hixon J, Johnson K, Chubukov V, et al. (January 2021). "AG-348 (Mitapivat), an allosteric activator of red blood cell pyruvate kinase, increases enzymatic activity, protein stability, and ATP levels over a broad range of PKLR genotypes". Haematologica. 106 (1): 238–249. doi:10.3324/haematol.2019.238865. PMC 7776327. PMID 31974203.
External links
- "Mitapivat sulfate". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03548220 for "A Study to Evaluate Efficacy and Safety of AG-348 in Not Regularly Transfused Adult Participants With Pyruvate Kinase Deficiency (PKD)" at ClinicalTrials.gov
- Clinical trial number NCT03559699 for "A Study Evaluating the Efficacy and Safety of AG-348 in Regularly Transfused Adult Participants With Pyruvate Kinase Deficiency (PKD)" at ClinicalTrials.gov