N-Acyl homoserine lactone
N-Acyl homoserine lactones (Abbreviated as AHLs or N-AHLs) are a class of signaling molecules involved in bacterial quorum sensing. Quorum sensing is a method of communication between bacteria that enables the coordination of group-based behavior based on population density. They signal changes in gene expression, such as switching between the flagella gene and the gene for pili for the development of a biofilm. Quorum sensing by the means of AHLs contributes to regulate the transcription of specific genes and therefore expression of specific phenotypes, including growth, virulence, biofilm formation, bioluminescence, production of exopolysaccharide (EPS). AHLs are used by over 50 gram-negative bacteria species (including several pathogenic species) use AHLs as autoinducers and the means of their communication in Quorum sensing.
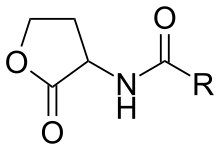
Formation
It arises by the reaction of acyl carrier proteins react with S-adenosylmethionine. The latter donates the equivalent of 4-aminobutyrolactone. Methylthioadenosine is a coproduct.[1]
Homoserine lactone is also a product of the proteolytic reaction of cyanogen bromide (CNBr) with a methionine residue. This reaction is important for chemical sequencing of proteins.
Structure
Main structural components of AHLs are the hydrophilic sections of homoserine lactone ring (biosynthesized from S-adenosylmethionine) and the central amide group, as well as the hydrophobic section strain-specific side hydrocarbon chain with varieties in length and level of oxygenation with a 3-oxo group. Hydrophilic sections are able to form hydrogen-bond network within the receptor binding site and the hydrophobic site contributes to diffusion through the cell membranes and binding within the hydrophobic pocket. The length of the acyl chain generally ranges from 4 to 18 carbons.
Following strategies were applied to disrupt the virulence programs of bacteria through antagonizing quorum sensing:
- quorum quenching: inhibiting the production or reducing the concentration of the autoinducers in the extracellular matrix
- Applying catalytic antibodies[2]
- interfering with the ligand-receptor interaction by adding antagonistic analogues[3]
The two critical factors in AHL-based QS are concentration and structure of AHL.
Signalling
In bacterial signalling, N-AHLs are produced within the bacterial cell and released into the environment. N-AHLs produced by different bacteria differ in the length of the R-group side-chain. Chain lengths vary from 4 to 18 carbon atoms and in the substitution of a carbonyl at the third carbon.[4]
Mechanism
In the process of quorum sensing, first LuxI protein synthesizes and catalyzes the formation of an acylated homoserin-lactone molecule. AHL can pass through cell membrane down the gradient to the environmental space. When the concentration of this autoinducer reaches a specific degree (quorum), cognate LuxR protein binds to the AHL and directs all regulate the transcription of the target genes. As a result, gene expression of an entire population will be coordinated.
Several studies have been investigating on the potential AHLs effective in infection and resistance to antibiotics. LuxR–LuxI system mediated by AHLs is the best screened QS system in multi-drug resistant bacteria species.
Determining the antibiotic susceptibility of P. aeruginosa, E. coli and K. pneumoniae species isolated from various clinics and showing different resistance profiles, investigating which signal molecules are effective in infection and elucidating the relationship between the signal molecules used and resistance profiles are among the main goals in preventing the problem of resistance.
Impacts of AHLs on Plants
Application of AHLs have been reported to activate the auxin-responsible GH3 promoter (upregulate auxin-related genes), and down-regulate the genes related to cytokinin (change in the ratio between auxin and cytokinin could promote the growth),[5] enhanced the nodulation in roots,[6] change in transpiration rate by increasing stomata opening and consequently higher water and mineral flow through the plant.[7]
See also
- Lactonase
Further reading
- Eberhard, A.; Burlingame, A. L.; Eberhard, C.; Kenyon, G. L.; Nealson, K. H.; Oppenheimer, N. J. (1981). "Structural identification of autoinducer of Photobacterium fischeri luciferase". Biochemistry. 20 (9): 2444–2449. doi:10.1021/bi00512a013. PMID 7236614. (discovery of homoserine lactone)
- Zhang Q, Li S, Hachicha M, Boukraa M, Soulère L, Efrit ML, Queneau Y. Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics. Molecules. 2021; 26(17):5135. https://doi.org/10.3390/molecules26175135
Notes
- Parveen, Nikhat; Cornell, Kenneth A. (2011). "Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism". Molecular Microbiology. 79 (1): 7–20. doi:10.1111/j.1365-2958.2010.07455.x. PMC 3057356. PMID 21166890.
- De Lamo Marin, Sandra; Xu, Yang; Meijler, Michael M.; Janda, Kim D. (March 2007). "Antibody catalyzed hydrolysis of a quorum sensing signal found in Gram-negative bacteria". Bioorganic & Medicinal Chemistry Letters. 17 (6): 1549–1552. doi:10.1016/j.bmcl.2006.12.118. ISSN 0960-894X.
- Palmer, Andrew G.; Senechal, Amanda C.; Mukherjee, Arijit; Ané, Jean-Michel; Blackwell, Helen E. (2014-06-18). "Plant Responses to Bacterial N-Acyl <scp>l</scp>-Homoserine Lactones are Dependent on Enzymatic Degradation to <scp>l</scp>-Homoserine". ACS Chemical Biology. 9 (8): 1834–1845. doi:10.1021/cb500191a. ISSN 1554-8929.
- Kumari, A.; Pasini, P.; Deo, S. K.; Flomenhoft, D.; Shashidhar, S.; Daunert, S. (2006). "Biosensing Systems for the Detection of Bacterial Quorum Signaling Molecules". Analytical Chemistry. 78 (22): 7603–7609. doi:10.1021/ac061421n. PMID 17105149.
- von Rad, Uta; Klein, Ilona; Dobrev, Petre I.; Kottova, Jana; Zazimalova, Eva; Fekete, Agnes; Hartmann, Anton; Schmitt-Kopplin, Philippe; Durner, Jörg (2008-09-03). "Response of Arabidopsis thaliana to N-hexanoyl-dl-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere". Planta. 229 (1): 73–85. doi:10.1007/s00425-008-0811-4. ISSN 0032-0935.
- Veliz-Vallejos, Debora F.; van Noorden, Giel E.; Yuan, Mengqi; Mathesius, Ulrike (2014-10-14). "A Sinorhizobium meliloti-specific N-acyl homoserine lactone quorum-sensing signal increases nodule numbers in Medicago truncatula independent of autoregulation". Frontiers in Plant Science. 5. doi:10.3389/fpls.2014.00551. ISSN 1664-462X.
- Palmer, Andrew G.; Senechal, Amanda C.; Mukherjee, Arijit; Ané, Jean-Michel; Blackwell, Helen E. (2014-06-18). "Plant Responses to Bacterial N-Acyl <scp>l</scp>-Homoserine Lactones are Dependent on Enzymatic Degradation to <scp>l</scp>-Homoserine". ACS Chemical Biology. 9 (8): 1834–1845. doi:10.1021/cb500191a. ISSN 1554-8929.