Neuroenhancement
Neuroenhancement or cognitive enhancement refers to the targeted enhancement and extension of cognitive and affective abilities based on an understanding of their underlying neurobiology in healthy persons who do not have any mental illness.[1][2][3][4][5][6] As such, it can be thought of as an umbrella term that encompasses pharmacological and non-pharmacological methods of improving cognitive, affective, and motor functionality, as well as the overarching ethico-legal discourse that accompanies these aims.[7] Critically, for any agent to qualify as a neuroenhancer, it must reliably engender substantial cognitive, affective, or motor benefits beyond normal functioning in healthy individuals, whilst causing few side effects: at most at the level of commonly used comparable legal substances or activities, such as caffeine, alcohol, and sleep-deprivation.
Pharmalogical neuroenhancement agents include the well-validated nootropics, such as citicoline,[8] Bacopa monnieri and phosphatidylserine,[6] as well as other drugs used for treating patients with neurological disorders. Non-pharmacological measures include non-invasive brain stimulation, which has been employed to improve various cognitive and affective functions, and brain-machine interfaces, which hold much potential to extend the repertoire of motor and cognitive actions available to humans.[9]
Although consideration of individual neuroenhancement agents is usually triggered by success in clinical and technological fields, they have also been used to attempt to help people with lack of normal cognitive, motor, and affective abilities: for example, social skills and empathy. In this case, neuroenhancement drugs try to increase oxytocin and decrease cortisol levels helping people better their communication and social interaction skills.[5]
Pharmacological
Modafinil
Modafinil is a wakefulness-promoting drug that decreases fatigue, increases vigilance, reduces daytime sleepiness, and improves mood.[4][5][10] Modafinil is currently licensed for treating patients with disorders such as narcolepsy, sleep apnea, and shift work sleep disorder.[2][5] This drug also seems promising in the treatment of depression and bipolar disorder.[5] Modafinil is currently being used by United States Air Force personnel for missions of great duration in an attempt to decrease fatigue amongst aircrew. It has become more popular amongst the general public. In an online poll conducted by Nature magazine, 8.8% of 1400 corresponding readers admitted use of modafinil for non-medical reasons. Their reasoning behind its use was for increasing concentration and focus on a specific task or to counteract sleep deficit and jetlag.[2] A comparison between the sales of modafinil to the number of patients revealed a disproportionate ratio, indicating high abuse.[2]
Modafinil has been reported to improve executive function in healthy non-sleep-deprived individuals, as well as potentially improving attention and learning and memory.[11] Effects on sleep deprived individuals are even more striking: a single dose resulted in enhanced wakefulness, executive functions, and memory.[10] In the case of sustained sleep deprivation, repeated intake of modafinil helped individuals maintain higher levels of wakefulness than the placebo, but did not help attention and executive function.[2][10] Since the majority of these trials were conducted on military personnel, further research needs to be conducted on the effects of modafinil on the general population. Modafinil may impair one's self-monitoring ability. A common trend found in research studies indicated that participants rated their performances on cognitive tests higher than it actually was, suggesting "overconfidence" effect.[2]
Modafinil is becoming increasingly popular among the general population.[7] Apart from a consumer's want to increase his neurological performance, there are financial incentives for manufacturers as well. Modafinil has a market share of more than $700 million a year, indicating a high degree of off-label use.[4] Modafinil is also one of the more easily available neuroenhancement drugs in the market today. Modafinil can be bought from many websites – mostly from Asian countries – as well as from darknet markets.[4][12][13] Modafinil first came into attention when world champion runner Kelli White was tested positive for illegally consuming modafinil in the Athletics World Championship in 2003, resulting in the loss of her two gold medals.[4]
Methylphenidate

Methylphenidate (MPH) is a stimulant that is used to treat attention-deficit hyperactivity disorder (ADHD). MPH is known to be highly abused by the general population, especially college students.[2][4] In an online poll conducted by Nature magazine, 12.4% of 1400 corresponding readers admitted use of MPH for non-medical reasons. Their reasoning behind its use was for increasing concentration, sleep deficit, and jetlag.[2]
A comparison between the sales of MPH to the number of patients revealed a disproportionate ratio, indicating high abuse.[2] MPH is believed to have a positive effect for memory consolidation, but studies have not been able to conclusively verify this claim.[2][10] Popular opinion that MPH enhances attention could not be verified.[2][10] Studies of MPH have reported improved problem solving skills. However, when these studies were repeated to replicate the results, the placebo group scored higher, indicating that MPH may even impair performance.[4]
These inconclusive, and generally negative, results for memory improvement are insufficient to explain the use of MPH for non-medical reasons. Users may have motives other than genuine neuroenhancement that propels its unprescribed use, such as subjective and recreational effects.[2] The lack of any result, positive or negative, indicated that the 10–20 mg dosage may be too low for the drug.[2] Further studies need to be conducted, looking at different doses of MPH.[2][10]
Memantine
Memantine is a NMDA receptor antagonist and is used to treat patients with moderate to severe Alzheimer's disease, but is also used as a neuroenhancement drug.[3] Studies conducted on memantine were unable to conclusively verify neuroenhancement capability of the drug. Since most of these studies were single dose tests of memantine, it is possible that these drugs would only show some effect, positive or negative, after continuous intake. Until then, single dose studies of memantine are not enough to reveal the drug's actual potential.[3]
Donepezil

Donepezil is an acetylcholinesterase inhibitor (AChEI) that is used to treat patients with mild to moderate Alzheimer's disease. While many AChEIs could be potential neuroenhancement substances, donepezil is the most commonly used AChEIs by the general population due to its widespread use for treating Alzheimer's disease.[3]
Most studies on donepezil are unable to conclusively verify the neuroenhancement capability of the drug.[3] In such studies, it was seen participants that took donepezil scored higher than those that took the placebo. Donepezil helps individuals retain training tasks, verbal memory, and episodic memory.[3] In sleep deprivation studies, while donepezil had no effect on well-rested patients, it had a positive effect on patients with 24 hours of sleep deprivation. Such patients benefited from increased memory performance and attention that would otherwise be deficit in such sleep-deprived conditions.[3] However, this effect was only seen in individuals whose performance declined significantly due to sleep deprivation.[3]
Non-pharmacological
Transcranial direct current stimulation
While neuroenhancement drugs are a potential method for cognitive performance enhancement, Transcranial direct current stimulation (tDCS) over the motor cortex (MC) is being seen as another potential method.[14] Although it was originally intended to help patients with brain injuries such as stroke, there has been a lot of interest in the last few years on tDCS's capabilities for healthy individuals as well. Recent studies have already shown improved neuroplasticity from tDCS to facilitate motor learning in young humans, and it may be possible to apply this method to the older segment of the workforce as well.[14]
Stimulating higher cognitive functions of the brain, such as the language function, with tDCS in one study resulted in improved word retrieval. tDCS works by enhancing the connectivity in a given stimulated network, providing neural efficiency in highly specific brain areas critical for task performance.[15] During this time, fMRI images also showed reduced activity in the semantic retrieval processes, suggesting more efficient processing in task-critical areas of the brain.[15] Reduced activity in circumscribed task-related areas has been attributed to consolidation of motor learning and superior memory performance. New research in tDCS is trying to localize the stimulation to affect the desired subset of highly specific task-relevant neurons.[15]
Deep brain stimulation

Deep brain stimulation (DBS) is another form of neuroenhancement. Unlike tDCS, though, DBS involves the implantation of a medical device, and is restricted for use for only a few, severe diseases such as Parkinson's disease and dystonia.[16] In one study, DBS improved movement by 39%, reduced disability by 38%, and improved quality of life by 30% for patients with dystonia over a course of 3 months.[16] The patients had a reduction in dystonia symptoms by 50%.[16] Improvement was noticeable within hours to days after DBS application. The benefits of DBS as of now are far more than those of high-dosage trihexyphenidyl, a powerful drug used in the treatment for dystonia.
Side effects
Neuroenhancements drugs are well tolerated by healthy humans.[2][3] These drugs are already in mainstream use to treat patients with different kinds of psychiatric disorders. Since most of the information on neuroenhancements and its capabilities are drawn from research experiments, the best way to determine adverse effects are drop-out rates and subjective rating.[2][3] The drop-out rates were minimal or non-existent for donepezil, memantine, MPH, and modafinil.[2][3] In the drug trials, participants reported the following adverse reactions to the consumption of donepezil, memantine, MPH, or modafinil: gastrointestinal complaints (nausea), headache, dizziness, nightmares, anxiety, drowsiness, nervousness, restlessness, sleep disturbances, and insomnia.[3] The side effects normally ceased in the course of treatment.[3] Although there were no reported side effects from DBS, 18% of the patients reported device-related complications such as infections due to lead dislodgment or breakage.[16]
Ethical, social and legal issues
Neuroenhancement is often seen analogous to the issue of doping in sports.[17] A common concern raised is an unfair advantage of people who consume enhancing drugs over people who don't. Many athletes, however, feel that the only way for them to win against athletes that take performance-enhancing drugs (PED) is for them to take PEDs as well; a similar thought process has developed within the general population in regard to people that consume neuroenhancement drugs.[17] In a research study of 18- to 34-year-olds, 50% of them had little or no objection to the concept of doping.[17] Students, in particular, often feel that cognitive neuroenhancers are acceptable.[17] However, parents and healthcare providers are concerned about the safety and well-being of those that consume neuroenhancement.[17] Generally, the moral acceptability (including fairness perceptions) of such substances for the purpose of neuroenhancement are an important factor in the decision to use or not use such drugs. Studies found that moral objections against such substances strongly decrease the willingness to use them.[18][19][20]
Neuroenhancement drugs play a key role in some recent novels and movies, such as Limitless (2011), probing and exploring the opportunities and threats of using neuro-enhancers in an imaginative way.[21]
In a recent article published by Jayne Lucke, the concept of neuroenhancement is compared to sildenafil. The author states that "recreational users of [sildenafil] had lower confidence in their ability to achieve an erection than non-users, even though they had significantly better erectile function. They become psychologically dependent on these drugs." The author believes a similar trend can be seen neuroenhancement users.[17] The author goes on to argue about how expectation, especially in students that are the general force, differs vastly on what is considered normal or good performance. Many argue that the only option for regulation of neuroenhancements is to allow it to everyone, thus minimizing cheating. Banning the drugs, on the other hand, may have detrimental consequences to society.[7] Not only would it create a black market, amplifying issues caused by illicit use, it would also increase the cost to society from enforcing the law.[17] Neuroenhancement drugs need to be assessed further for their merits and adverse effects, making it easier for policy makers to make a call on the regulation of such drugs.[10]
Scope for cognitive enhancement
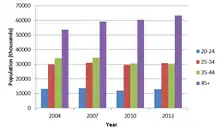
Proponents of cognitive enhancement have argued that there are vast potential benefits for the workforce, especially for the older segment.[14] Due to advances in medical technology over the last century, the human life expectancy has increased significantly. Demographics for developed countries indicate rapid growth of the older segment of the workforce. Advancing age generally shows a pattern in the reduction of the ability to acquire new skills, but integration in the industry today requires employees to be able to acquire and retain new skills more than ever before.[14]
Opinion
General public
The opinion of the general public on the issue of neuroenhancement is scattered.[17][22] In general, the younger population under the age of 25 feel that neuroenhancements are acceptable or that the decision lies in the hand of that individual. Healthcare officials and parents feel concerned due to safety factors, lack of complete information on these drugs, and possible irreversible adverse effects.[17] Such concerns have been shown to reduce the willingness to take such drugs.[23][24][19]
A recent German study among 6.454 employees found a rather low life-time prevalence of cognitive enhancement drug use (namely 2.96%), while the willingness to take such drugs was found in every tenth respondent (10.45%).[25] Studies have estimated that between 7–9% of the college population in the United States consumes neuroenhancement drugs. Some studies estimate this figure to be as high as 12% or even 20%.[22] A large-scale survey using a random sample of more than 5.000 German university students found a relatively low 30-days prevalence of 1.2%, 2.3% indicated the use of such drugs within the last 6 months, 3.2% within the last 12 months and during 4.6% during their lifetime, respectively.[23] Of those students, who used such substances during the last 6 months, 39.4% reported their use once in this period, 24.2% twice, 12.1% three times and 24.2% more than three times. It has been shown that consumers of neuroenhancement drugs are much more willing to also use them in the future, e.g. due to positive experiences or a tendency towards addiction.[20][26] Students primarily attribute consumption of these drugs for increased concentration, improved alertness, or to "get high".[17][22] Neuroenhancement drug users rated the positive potential of neuroenhancement drugs higher than non-users, and rated the adverse effects of these drugs lower than non-users, showing more confidence in the result of these drugs. In a survey of 1324 German students, 32% of participants that do not consume neuroenhancement drugs felt they had positive cognitive effects while 12% felt they had a relaxation effect.[22] In contrast, 54% of participants that do consume neuroenhancement drugs felt they had a positive cognitive effect while 25% felt they had a relaxation effect.

The need to remain "alert" and "focused" can also been seen in the trend of caffeine consumption. The caffeine consumption for both students and the general population of the US is around 90%.[22][27] Students who consume neuroenhancements also had a higher frequency of consuming psychoactive lifestyle drugs such as cannabis. This demonstrates a trend of psychological addiction amongst neuroenhancement users.[22]
A study among German university teachers (including professors) found a very low prevalence of neuroenhancement drug use.[20] Only 0.9% of the respondents reported the use of such drugs. However, 10% of the respondents are willing to take such drugs in the future, what might indicate a potential increase of the prevalence. One reason to use such drugs was work-related stress.
Physicians
Physicians play an important role in determining the potential abuse of neuroenhancing drugs. While some neuroenhancing drugs do not require a prescription and are easily available, others that require prescription are up to the discretion of the physician. In a survey conducted among Swiss psychiatrists and general practitioners, the majority of surveyed physicians agreed that their criteria to determine whether or not a dysfunction should be considered a disease is if the patient indicates subjective suffering and/or negative consequences for everyday ability to work.[6] The surveyed physicians, however, were in majority agreement that they do not prescribe medication without a clear indication of such a dysfunction.[6]
See also
- Cognitive liberty
- Evidence-based learning
- List of drugs used by militaries
- Intelligence amplification
References
- Battleday, Ruairidh; Brem, Anna-Katharine (28 July 2015). "Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review". European Neuropsychopharmacology. 25 (11): 1865–1881. doi:10.1016/j.euroneuro.2015.07.028. PMID 26381811. S2CID 23319688.
- Repantis, Dimitris; Schlattmann, Peter (2010). "Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review". Pharmacological Research. 62 (3): 187–206. doi:10.1016/j.phrs.2010.04.002. PMID 20416377.
- Repantis, Dimitris (June 2010). "Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review". Pharmacological Research. 61 (6): 473–481. doi:10.1016/j.phrs.2010.02.009. PMID 20193764.
- Normann, Claus; Berger, Mathias (November 2008). "Neuroenhancement: status quo and perspectives" (PDF). European Archives of Psychiatry and Clinical Neuroscience. 258: 110–114. doi:10.1007/s00406-008-5022-2. PMID 18985306. S2CID 9733191.
- Normann, Claus; Nissen, C (November 2012). "Neuroenhancement strategies for psychiatric disorders: rationale, status quo and perspectives". European Archives of Psychiatry and Clinical Neuroscience. 262: 113–116. doi:10.1007/s00406-012-0356-1. PMID 22932721. S2CID 42536705.
- Ott, R. (2012). "Neuroenhancement - perspectives of Swiss psychiatrists and general practitioners". Swiss Medical Weekly. 142: w13707. doi:10.4414/smw.2012.13707. PMID 23254869.
- Veit, Walter (2018). "Cognitive Enhancement and the Threat of Inequality". Journal of Cognitive Enhancement. 2 (4): 404–410. doi:10.1007/s41465-018-0108-x. S2CID 158643005.
- Tardner, P. (2020-08-30). "The use of citicoline for the treatment of cognitive decline and cognitive impairment: A meta-analysis of pharmacological literature • International Journal of Environmental Science & Technology". International Journal of Environmental Science & Technology. Retrieved 2020-08-31.
- Nair, Prashant (2013-11-12). "Brain–machine interface". Proceedings of the National Academy of Sciences. 110 (46): 18343. Bibcode:2013PNAS..11018343N. doi:10.1073/pnas.1319310110. ISSN 0027-8424. PMC 3831969. PMID 24222678.
- Ragan, Ian; Bard, I; Singh, I; Independent Scientific Committee on Drugs (February 2013). "What should we do about student use of cognitive enhancers? An analysis of current evidence". Neuropharmacology. 64: 588–595. doi:10.1016/j.neuropharm.2012.06.016. PMID 22732441. S2CID 207227699.
- Battleday, R.M.; Brem, A.-K. (Nov 2015). "Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review". European Neuropsychopharmacology. 25 (11): 1865–1881. doi:10.1016/j.euroneuro.2015.07.028. PMID 26381811. S2CID 23319688.
- Woolf, Nicky (31 May 2015). "Silk Road sentencing: why governments can't win the war on darknet drugs". The Guardian. Retrieved 30 December 2016.
- Valette, Jean-Jacques (9 September 2016). "Du commerce illicite à la liberté d'expression totale, on a plongé dans le darknet". We Demain. Retrieved 30 December 2016.
- Zimerman, Maximo; Nitsch, M; Giraux, P; Gerloff, C; Cohen, LG; Hummel, FC (2013). "Neuroenhancement of the Aging Brain: Restoring Skill Acquisition in Old Subjects". Annals of Neurology. 73 (1): 10–15. doi:10.1002/ana.23761. PMC 4880032. PMID 23225625.
- Meinzer, Marcus; Antonenko, D; Lindenberg, R; Hetzer, S; Ulm, L; Avirame, K; Flaisch, T; Flöel, A (2012). "Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation". The Journal of Neuroscience. 32 (5): 1859–1866. doi:10.1523/JNEUROSCI.4812-11.2012. PMC 6703352. PMID 22302824.
- Kupsch, Andreas; Benecke, Reiner; Müller, Jörg; Trottenberg, Thomas; Schneider, Gerd-Helge; Poewe, Werner; Eisner, Wilhelm; Wolters, Alexander; Müller, Jan-Uwe; Deuschl, Günther; Pinsker, Marcus O.; Skogseid, Inger Marie; Roeste, Geir Ketil; Vollmer-Haase, Juliane; Brentrup, Angela; Krause, Martin; Tronnier, Volker; Schnitzler, Alfons; Voges, Jürgen; Nikkhah, Guido; Vesper, Jan; Naumann, Markus; Volkmann, Jens; Deep-Brain Stimulation for Dystonia Study Group (2006). "Pallidal Deep-Brain Stimulation in Primary Generalized or Segmental Dystonia". New England Journal of Medicine. 355 (19): 1978–1990. doi:10.1056/NEJMoa063618. PMID 17093249.
- Lucke, Jayne C.; Bell, Stephanie K.; Patridge, Bradley J.; Hall, Wayne D. (2011). "Academic Doping or Viagra for the brain?". EMBO Rep. 12 (3): 197–201. doi:10.1038/embor.2011.15. PMC 3059919. PMID 21311560.
- Sattler, S., Sauer, C., Mehlkop, G., Graeff, P. (2013). "The Rationale for Consuming Cognitive Enhancement Drugs in University Students and Teachers". PLOS ONE. 8 (7): e68821. Bibcode:2013PLoSO...868821S. doi:10.1371/journal.pone.0068821. PMC 3714277. PMID 23874778.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Sattler, S., Forlini, C., Racine, E., Sauer, C. (2013). "Impact of Contextual Factors and Substance Characteristics on Perspectives toward Cognitive Enhancement". PLOS ONE. 8 (8): e71452. Bibcode:2013PLoSO...871452S. doi:10.1371/journal.pone.0071452. PMC 3733969. PMID 23940757.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Wiegel C., Sattler S., Göritz A. S. (2015). "Work-related stress and cognitive enhancement among university teachers". Anxiety, Stress & Coping. 29 (1): 1–18. doi:10.1080/10615806.2015.1025764. PMID 25747817. S2CID 22273733.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Zwart H. (2014). "Limitless as a neuro-pharmaceutical experiment and as a Daseinsanalyse: on the use of fiction in preparatory debates on cognitive enhancement" (PDF). Medicine, Health Care and Philosophy. 17 (1): 29–38. doi:10.1007/s11019-013-9481-5. PMID 23585022. S2CID 29893291.
- Eickenhorst, Patrick; Vitzthum, Karin; Klapp, Burghard F.; Groneberg, David; Mache, Stefanie (2012). "Neuroenhancement Among German University Students: Motives, Expectations, and Relationship with Psychoactive Lifestyle Drugs". Journal of Psychoactive Drugs. 44 (5): 418–427. doi:10.1080/02791072.2012.736845. PMID 23457893. S2CID 6621896.
- Sattler S., Wiegel C. (2013). "Cognitive test anxiety and cognitive enhancement: the influence of students' worries on their use of performance-enhancing drugs". Substance Use and Misuse. 48 (3): 220–32. doi:10.3109/10826084.2012.751426. PMID 23302063. S2CID 34698382.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Sattler S., Sauer C., Mehlkop G., Graeff P. (2013). "The Rationale for Consuming Cognitive Enhancement Drugs in University Students and Teachers". PLOS ONE. 8 (7): e68821. Bibcode:2013PLoSO...868821S. doi:10.1371/journal.pone.0068821. PMC 3714277. PMID 23874778.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Sattler S., Schunck R. (2016). "Associations Between the Big Five Personality Traits and the Non-Medical Use of Prescription Drugs for Cognitive Enhancement". Frontiers in Psychology. 6: 1971. doi:10.3389/fpsyg.2015.01971. PMC 4700267. PMID 26779083.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Sattler, S., Mehlkop, G., Graeff, P., Sauer, C. (2014). "Evaluating the drivers of and obstacles to the willingness to use cognitive enhancement drugs: the influence of drug characteristics, social environment, and personal characteristics". Substance Abuse Treatment, Prevention, and Policy. 9: 8. doi:10.1186/1747-597X-9-8. PMC 3928621. PMID 24484640.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Olsen, Nicole (2013). "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students". Honors Theses.
Further reading
- Blank, Robert H. (5 November 2016). Cognitive Enhancement: Social and Public Policy Issues. ISBN 978-1-349-84797-6.
- Bostrom, Nick (2016). Superintelligence: Paths, Dangers, Strategies. Oxford University Press. ISBN 9780198739838.
- Bostrom, Nick; Sandberg, Anders (2009). "Cognitive Enhancement: Methods, Ethics, Regulatory Challenges". Science and Engineering Ethics. 15 (3): 311–341. doi:10.1007/s11948-009-9142-5. PMID 19543814. S2CID 6846531.
- Veit, Walter (2018). "Cognitive Enhancement and the Threat of Inequality". Journal of Cognitive Enhancement. 2 (4): 404–410. doi:10.1007/s41465-018-0108-x. S2CID 158643005.