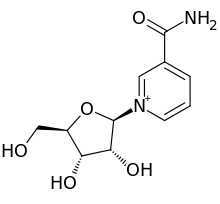
Nicotinamide riboside
Nicotinamide riboside (NR, SR647) is a pyridine-nucleoside similar to vitamin B3, functioning as a precursor to nicotinamide adenine dinucleotide or NAD+.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium | |
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H15N2O5+ |
| Molar mass | 255.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemistry
While the molecular weight of nicotinamide riboside is 255.25 g/mol,[2] that of its chloride salt is 290.70 g/mol.[3][4] As such, 100 mg of nicotinamide riboside chloride provides 88 mg of nicotinamide riboside. Recently, the crystal structure of a new series of crystalline forms of other nicotinamide riboside salts has been discovered. These salts correspond to different enantiomers of hydrogen tartrate and hydrogen malate and have a molecular weight of 404.33 and 388.33 g/mol respectively.[5]
History
Nicotinamide riboside (NR) was first described in 1944 as a growth factor, termed Factor V, for Haemophilus influenza, a bacterium that lives in and depends on blood. Factor V, purified from blood, was shown to exist in three forms: NAD+, NMN and NR. NR was the compound that led to the most rapid growth of this bacterium.[6] H. influenza cannot grow on nicotinic acid, nicotinamide, tryptophan or aspartic acid, which were the previously known precursors of NAD+.[7]
In 2000, yeast Sir2 was shown to be an NAD+-dependent protein lysine deacetylase,[8] which led several research groups to probe yeast NAD+ metabolism for genes and enzymes that might regulate lifespan.[9]
Synthesis
Different biosynthetic pathways are responsible for converting the different B3 vitamins into NAD+. The enzyme nicotinamide phosphoribosyltransferase (Nampt) catalyzes the rate-limiting step of the two-step pathway converting nicotinamide to NAD+.[10] NR kinase enzymes can also function as a salvage pathway for NAD+, but this pathway is not essential.[10]
Commercialization
ChromaDex licensed patents in July 2012, and began to develop a process to bring NR to market.[11] ChromaDex has been in a patent dispute with Elysium Health over the rights to nicotinamide riboside supplements.[12]
Biosynth Carbosynth licensed a patent to produce new NR salts WO 2021013795. The new carboxylic acid salts in crystalline form can be used in nutritional supplements and pharmaceutical compositions.
Research
A phase 1 clinical trial in newly diagnosed people with Parkinson's disease showed that nicotinamide riboside supplements boosted levels of brain NAD.[13][14] A larger clinical trial is underway to confirm the results.[15][16]
See also
- Nicotinamide mononucleotide
- Niacin
- Nicotinamide
- Vitamin B3
- Nicotinamide adenine dinucleotide
- Sirtuin
- Poly (ADP-ribose) polymerase
References
- Bogan, K.L., Brenner, C. (2008). "Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition". Annu. Rev. Nutr. 28: 115–130. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Nicotinamide riboside". pubchem.ncbi.nlm.nih.gov.
- "GRAS Notices, GRN No. 635". www.accessdata.fda.gov. Retrieved 18 February 2019.
- "Spherix/ChromaDex GRAS submission" (PDF). FDA.gov. Retrieved 18 February 2019.
- Günter, S; et al. (2021). "New Crystalline Salts of Nicotinamide Riboside as Food Additives". Molecules. MDPI. 26 (9): 2729. doi:10.3390/molecules26092729. PMC 8125264. PMID 34066468.
- Gingrich, W; Schlenk, F (June 1944). "Codehydrogenase I and Other Pyridinium Compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae". Journal of Bacteriology. 47 (6): 535–50. doi:10.1128/JB.47.6.535-550.1944. PMC 373952. PMID 16560803.
- Belenky, P.; et al. (2007). "NAD+ Metabolism in Health and Disease". Trends in Biochemical Sciences. 32 (1): 12–19. doi:10.1016/j.tibs.2006.11.006. PMID 17161604.
- Imai, S.; et al. (2000). "Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase". Nature. 403 (6771): 795–800. Bibcode:2000Natur.403..795I. doi:10.1038/35001622. PMID 10693811. S2CID 2967911.
- Anderson; et al. (2003). "Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae". Nature. 423 (6936): 181–185. Bibcode:2003Natur.423..181A. doi:10.1038/nature01578. PMC 4802858. PMID 12736687.
- Fletcher RS, Lavery GG (2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (1): R107–R121. doi:10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- "ChromaDex Licenses Exclusive Patent Rights for Nicotinamide Riboside (NR) Vitamin Technologies". 2012-07-16. Retrieved 15 February 2019.
- Melody M. Bomgardner (2018). "Firms feud over purported age-fighting molecule". Chemical & Engineering News. 96 (33).
- "Vitamin B3 supplement shows early promise for Parkinson's". Parkinson's UK. Retrieved 2022-09-11.
- Brakedal, Brage; Dölle, Christian; Riemer, Frank; Ma, Yilong; Nido, Gonzalo S.; Skeie, Geir Olve; Craven, Alexander R.; Schwarzlmüller, Thomas; Brekke, Njål; Diab, Joseph; Sverkeli, Lars; Skjeie, Vivian; Varhaug, Kristin; Tysnes, Ole-Bjørn; Peng, Shichun (2022-03-01). "The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease". Cell Metabolism. 34 (3): 396–407.e6. doi:10.1016/j.cmet.2022.02.001. ISSN 1550-4131. PMID 35235774.
- Bailey, Suzanne (2022-03-07). "Nicotinamide riboside for Parkinson's: pilot results published". Cure Parkinson's. Retrieved 2022-09-11.
- Haukeland University Hospital (2020-06-05). "A Randomized Controlled Trial of Nicotinamide Supplementation in Early Parkinson's Disease: the NOPARK Study".
{{cite journal}}: Cite journal requires|journal=(help)