Original antigenic sin
Original antigenic sin, also known as antigenic imprinting, the Hoskins effect,[1] or immunological imprinting,[2] is the propensity of the immune system to preferentially use immunological memory based on a previous infection when a second slightly different version of that foreign pathogen (e.g. a virus or bacterium) is encountered. This leaves the immune system "trapped" by the first response it has made to each antigen, and unable to mount potentially more effective responses during subsequent infections. Antibodies or T-cells induced during infections with the first variant of the pathogen are subject to repertoire freeze, a form of original antigenic sin.
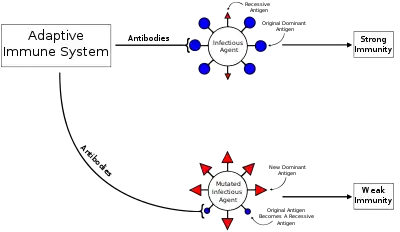
The phenomenon has been described in relation to influenza virus, SARS-CoV-2,[2] dengue fever, human immunodeficiency virus (HIV) [3] and to several other viruses.[4]
History
This phenomenon was first described in 1960 by Thomas Francis Jr. in the article "On the Doctrine of Original Antigenic Sin".[5][6] It is named by analogy to the Christian theological concept of original sin. According to Francis as cited by Richard Krause:[6]
"The antibody of childhood is largely a response to dominant antigen of the virus causing the first type A influenza infection of the lifetime. [...] The imprint established by the original virus infection governs the antibody response thereafter. This we have called the Doctrine of the Original Antigenic Sin."
In B cells
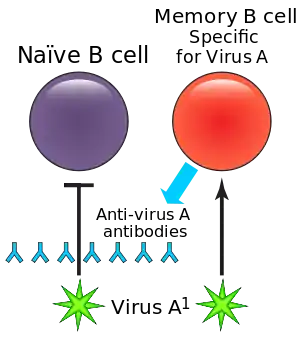
During a primary infection, long-lived memory B cells are generated, which remain in the body and protect from subsequent infections. These memory B cells respond to specific epitopes on the surface of viral proteins to produce antigen-specific antibodies and can respond to infection much faster than naive B cells can to novel antigens. This effect lessens time needed to clear subsequent infections.
Between primary and secondary infections or following vaccination, a virus may undergo antigenic drift, in which the viral surface proteins (the epitopes) change through natural mutation. This allows the virus to escape the immune system. The altered virus preferentially reactivates previously activated high-affinity memory B cells and spurs antibody production. However, the antibodies produced generally ineffectively bind to the altered epitopes. In addition, these antibodies inhibit activation of naive B cells that could make more effective antibodies to the second virus. This leads to a less effective immune response and recurrent infections may take longer to clear.[7]
Original antigenic sin has important implications for vaccine development.[8] In dengue fever, for example, once a response against one serotype has been established, it is unlikely that vaccination against a second will be effective. This implies that balanced responses against all four virus serotypes must be established with the first vaccine dose.[9]
Activation of naive B cells that recognize novel epitopes may be attentuated with repeated infection with variant influenza viruses.[10] However, the impact of antigenic sin on protection has not been well established and appears to differ with each infectious agent vaccine, geographic location, and age.[7] Research done in 2011 found reduced antibody responses to the 2009 pandemic H1N1 influenza vaccine in individuals who had been vaccinated against the seasonal A/Brisbane/59/2007 (H1N1) within the previous three months.[8]
In cytotoxic T cells
A similar phenomenon has been described in cytotoxic T cells (CTL).[11] It has been demonstrated that during a second infection by a different strain of dengue virus, the CTLs prefer to release cytokines instead of causing cell lysis. As a result, the production of these cytokines is thought to increase vascular permeability and exacerbate damage to endothelial cells, resulting in dengue hemorrhagic fever.[12]
Several groups have attempted to design vaccines for HIV and hepatitis C based on induction of CTL response. The finding that the CTL response may be biased by original antigenic sin may help to explain the limited effectiveness of these vaccines. Viruses like HIV are highly variable and undergo mutation frequently; due to original antigenic sin, HIV infection induced by viruses that express slightly different epitopes (than those in a viral vaccine) might fail to be controlled by the vaccine. It has been hypothesized that: if original antigenic sin is a common phenomenon, a naively designed single-component vaccine could conceivably make an infection even worse than if no vaccination at all had occurred. The hypothesized mechanism is that the immune response would be "trapped" in a less effective response. Therefore a recommendation was made for vaccines with multiple components or that target conserved epitopes.[11]
See also
- Antibody-dependent enhancement
- Cell mediated immunity
- Genomic imprinting
- Humoral immunity
- Polyclonal response
- Local optimum
References
- Hoskins, T.W.; Davies, Joan R.; Smith, A.J.; Miller, Christine L.; Allchin, Audrey (1979). "Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital". The Lancet. 313 (8106): 33–35. doi:10.1016/s0140-6736(79)90468-9.
- Focosi, Daniele; Genoni, Angelo; Lucenteforte, Ersilia; Tillati, Silvia; Tamborini, Antonio; Spezia, Pietro Giorgio; Azzi, Lorenzo; Baj, Andreina; Maggi, Fabrizio (2021-04-01). "Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin". Life. 11 (4): 298. doi:10.3390/life11040298. ISSN 2075-1729. PMC 8067214. PMID 33915711.
- Singh, Rana AK; Rodgers, John R; Barry, Michael A (2002). "The role of T cell antagonism and original antigenic sin in genetic immunization" (PDF). The Journal of Immunology. 169 (12): 6779–6786. doi:10.4049/jimmunol.169.12.6779. PMID 12471109. Retrieved May 14, 2021.
- Deem, Michael W.The Adaptive Immune Response Archived 2008-07-04 at the Wayback Machine Rice University
- Thomas Francis Jr (1960). "On the doctrine of original antigenic sin". Proceedings of the American Philosophical Society. 104 (6): 572–578. JSTOR 985534.
- Krause R (2006). "The swine flu episode and the fog of epidemics". Emerg Infect Dis. 12 (1): 40–43. doi:10.3201/eid1201.051132. PMC 3291407. PMID 16494715.
- Lambert PH, Liu M, Siegrist CA (2005). "Can successful vaccines teach us how to induce efficient protective immune responses?". Nat Med. 11 (4 Suppl): S54–62. doi:10.1038/nm1216. PMID 15812491. S2CID 11685892.
- Choi, Yoon Seok; Baek, Yun Hee; Kang, Wonseok; et al. (September 2011). "Reduced Antibody Responses to the Pandemic (H1N1) 2009 Vaccine after Recent Seasonal Influenza Vaccination". Clinical and Vaccine Immunology. 18 (9): 1519–1523. doi:10.1128/CVI.05053-11. PMC 3165229. PMID 21813667.
- Midgley, Claire M.; Bajwa-Joseph, Martha; Vasanawathana, Sirijitt; et al. (January 2011). "An In-Depth Analysis of Original Antigenic Sin in Dengue Virus Infection". Journal of Virology. 85 (1): 410–421. doi:10.1128/JVI.01826-10. PMC 3014204. PMID 20980526.
- Kim, J.H.; Skountzou, I.; Compans, R.; Jacob, J. (1 September 2009). "Original antigenic sin responses to influenza viruses". Journal of Immunology. 183 (5): 3294–301. doi:10.4049/jimmunol.0900398. PMC 4460008. PMID 19648276.
- McMichael AJ (1998). "The original sin of killer T cells". Nature. 394 (6692): 421–422. doi:10.1038/28738. PMID 9697760.
- Juthathip Mongkolsapaya (2006). "T Cell Responses in Dengue Hemorrhagic Fever: Are Cross-Reactive T Cells Suboptimal?". J. Immunol. 176 (6): 3821–3829. doi:10.4049/jimmunol.176.6.3821. PMID 16517753.