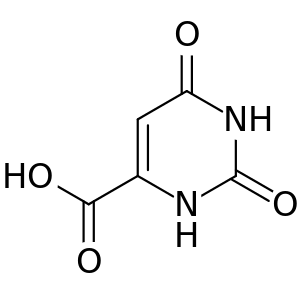
Orotic acid
Orotic acid is a pyrimidinedione and a carboxylic acid. Historically it was believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.563 |
| Chemical and physical data | |
| Formula | C5H4N2O4 |
| Molar mass | 156.097 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The compound is synthesized in the body via a mitochondrial enzyme, dihydroorotate dehydrogenase[1] or a cytoplasmic enzyme of pyrimidine synthesis pathway. It is sometimes used as a mineral carrier in some dietary supplements (to increase their bioavailability), most commonly for lithium orotate.
Synthesis
Dihydroorotate is synthesized to orotic acid by the enzyme dihydroorotate dehydrogenase, where it later combines with phosphoribosyl pyrophosphate (PRPP) to form orotidine-5'-monophosphate (OMP). A distinguishing characteristic of pyrimidine synthesis is that the pyrimidine ring is fully synthesized before being attached to the ribose sugar, whereas purine synthesis happens by building the base directly on the sugar.[2]
Pathology
A buildup of orotic acid can lead to orotic aciduria and acidemia. It may be a symptom of an increased ammonia load due to a metabolic disorder, such as a urea cycle disorder.
In ornithine transcarbamylase deficiency, an X-linked inherited and the most common urea cycle disorder, excess carbamoyl phosphate is converted into orotic acid. This leads to an increased serum ammonia level, increased serum and urinary orotic acid levels and a decreased serum blood urea nitrogen level. This also leads to an increased urinary orotic acid excretion, because the orotic acid is not being properly utilized and must be eliminated. The hyperammonemia depletes alpha-ketoglutarate leading to the inhibition of the tricarboxylic acid cycle (TCA) decreasing adenosine triphosphate (ATP) production.
Orotic aciduria is a cause of megaloblastic anaemia.
See also
References
- Rawls J, Knecht W, Diekert K, Lill R, Löffler M (April 2000). "Requirements for the mitochondrial import and localization of dihydroorotate dehydrogenase". European Journal of Biochemistry. 267 (7): 2079–87. doi:10.1046/j.1432-1327.2000.01213.x. PMID 10727948.
- Harvey D, Ferrier D, eds. (2008). Biochemistry (PDF) (5th ed.). Lippincott, Williams & Wilkins. p. 302.
Further reading
- Greenbaum SB (1954). "Potential Metabolic Antagonists of Orotic Acid: 6-Uracilsulfonamide and 6-Uracil Methyl Sulfone". Journal of the American Chemical Society. 76 (23): 6052–6054. doi:10.1021/ja01652a056.
External links
- Orotic+Acid at the US National Library of Medicine Medical Subject Headings (MeSH)