Itruvone
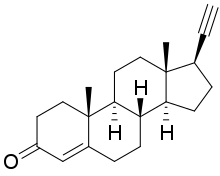
Itruvone (INN; developmental code name PH10), also known as pregn-4-en-20-yn-3-one, is a vomeropherine which is under development by VistaGen Therapeutics as a nasal spray for the treatment of major depressive disorder.[1][2][3][4][5][6]
 | |
| Clinical data | |
|---|---|
| Other names | PH10; PH10A; PH10 NS; Pregn-4-en-20-yn-3-one |
| Routes of administration | Intranasal (spray) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H28O |
| Molar mass | 296.454 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- "PH-10 Nasal Spray". AdisInsight. Springer Nature Switzerland AG.
- Monti L, Liebowitz MR (February 2022). "Neural circuits of anxiolytic and antidepressant pherine molecules". CNS Spectr. 27 (1): 66–72. doi:10.1017/S109285292000190X. PMID 33092667. S2CID 225053518.
- "VistaGen Therapeutics Acquires Worldwide Rights to Develop and Commercialize PH10, a First-in-Class Intranasally Administered Neuroactive Steroid with Rapid-onset Antidepressant Effects for Major Depressive Disorder Demonstrated in Phase 2a Study :: VistaGen Therapeutics, Inc. (VTGN)". VistaGen Therapeutics, Inc. Retrieved 2019-12-19.
- "Health Care Digest: A small biotech goes head first, Gilead's lower tax rate and more". www.bizjournals.com. San Francisco Business Times. October 29, 2018. Retrieved 20 July 2020.
{{cite web}}: CS1 maint: url-status (link) - Liebowitz MR, Nicolini H, Monti L, Hanover R (2013). "PH 10 may be a new rapidly acting intranasally administered antidepressant". American Society of Clinical Psychopharmacology (ASCP) Annual Meeting, Miami, FL.
{{cite journal}}: Cite journal requires|journal=(help) - Jancin B (2013). "Novel intranasal antidepressant shows results after 1 week". Clinical Psychiatry News. Retrieved 13 January 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.