Pafolacianine
Pafolacianine, sold under the brand name Cytalux, is an optical imaging agent used in fluorescence-guided surgery.[1][2][3][4][5]
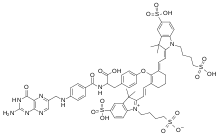 | |
| Clinical data | |
|---|---|
| Trade names | Cytalux |
| Other names | OTL38, OTL-0038 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C61H67N9O17S4 |
| Molar mass | 1326.49 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects of pafolacianine include infusion-related reactions, including nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, itching and hypersensitivity.[2][3]
It was approved for medical use in the United States in November 2021.[2][6][5]
Pafolacianine is a fluorescent drug that targets the folate receptor alpha (FRa).[1]
Medical uses
Pafolacianine is indicated as an adjunct for intraoperative identification of malignant lesions in people with ovarian cancer.[1][2]
History
The safety and effectiveness of pafolacianine was evaluated in a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer who were scheduled to undergo surgery.[2][3] Of the 134 women (ages 33 to 81 years) who received a dose of pafolacianine and were evaluated under both normal and fluorescent light during surgery, 26.9% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.[2][3]
The U.S. Food and Drug Administration (FDA) granted the application for pafolacianine orphan drug, priority review, and fast track designations.[2][3][7] The FDA granted the approval of Cytalux to On Target Laboratories, LLC.[2]
Figures

References
- "Cytalux- pafolacianine injection injection". DailyMed. Retrieved 18 December 2021.
- "FDA Approves New Imaging Drug to Help Identify Ovarian Cancer Lesions". U.S. Food and Drug Administration (FDA) (Press release). 29 November 2021. Retrieved 30 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves pafolacianine for identifying malignant ovarian cancer lesions". U.S. Food and Drug Administration (FDA). 1 December 2021. Retrieved 2 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Predina JD, Newton AD, Connolly C, Dunbar A, Baldassari M, Deshpande C, et al. (February 2018). "Identification of a Folate Receptor-Targeted Near-Infrared Molecular Contrast Agent to Localize Pulmonary Adenocarcinomas". Molecular Therapy. 26 (2): 390–403. doi:10.1016/j.ymthe.2017.10.016. PMC 5835020. PMID 29241970.
- Lauwerends LJ, Abbasi H, Bakker Schut TC, Van Driel PB, Hardillo JA, Santos IP, et al. (June 2022). "The complementary value of intraoperative fluorescence imaging and Raman spectroscopy for cancer surgery: combining the incompatibles". European Journal of Nuclear Medicine and Molecular Imaging. 49 (7): 2364–2376. doi:10.1007/s00259-022-05705-z. PMC 9165240. PMID 35102436.
- "On Target Laboratories Announces FDA Approval of Cytalux (pafolacianine) injection for Identification of Ovarian Cancer During Surgery". On Target Laboratories. 29 November 2021. Retrieved 30 November 2021 – via PR Newswire.
- "Pafolacianine Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 23 December 2014. Retrieved 30 November 2021.
- Predina JD, Newton AD, Xia L, Corbett C, Connolly C, Shin M, et al. (March 2018). "An open label trial of folate receptor-targeted intraoperative molecular imaging to localize pulmonary squamous cell carcinomas". Oncotarget. 9 (17): 13517–13529. doi:10.18632/oncotarget.24399. PMC 5862595. PMID 29568374.
External links
- "Pafolacianine". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03180307 for "OTL38 for Intra-operative Imaging of Folate Receptor Positive Ovarian Cancer" at ClinicalTrials.gov