Paramecium biaurelia
Paramecium biaurelia is a species of unicellular ciliates under the genus Paramecium, and one of the cryptic species of Paramecium aurelia.[2] It is a free-living protist in water bodies and harbours several different bacteria as endosymbionts.[3][4][5] Although the bacteria are parasites by definition, they also exhibit mutual relationship with the protist by providing survival benefits.[6] It is used as an organism model in the study of the effects of gravitational forces in different environments.[7][8][9]
| Paramecium biaurelia | |
|---|---|
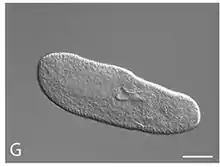 | |
| Paramecium biaurelia with large nucleus and smaller vacuole containing bacteria | |
| Scientific classification | |
| Kingdom: | Chromista |
| Superphylum: | Alveolata |
| Phylum: | Ciliophora |
| Class: | Oligohymenophorea |
| Order: | Peniculida |
| Family: | Parameciidae |
| Genus: | Paramecium |
| Species: | P. biaurelia |
| Binomial name | |
| Paramecium biaurelia Sonneborn, 1975[1] | |
Paramecium biaurelia was described by Tracy Morton Sonneborn in 1975 while analysing the different cryptic species of P. aurelia.[1]
Biology
Paramecium biaurelia is a unicellular protist with elongated body, which measures about 133 μm in length. The distinguishing feature from other species of P. aurelia is that it reproduces at below 21°C and specifically between 9 PM and 7 AM.[10] It is also genetically distinct (clade) without any indication of genetic mixing (gene flow) with other cryptic species.[11]
The most important bacterial endosymbionts are Holospora caryophila,[12] Caedibacter paraconjugatus,[5] and (Candidatus) Bealeia paramacronuclearis, which are members of the family Rickettsiaceae, and (Candidatus) Fokinia cryptica, which belongs to the family Midichloriaceae.[4] The bacteria are gram-negative species. They are energy parasites as they depend on the host's ATP for their energetic functions, but they increase the growth rate of the protists in return.[6] H. caryophila can infect P. biaurelia and P. caudatum, and was reclassified into a new genus as Preeria caryophila, based on detailed morphological analysis and phylogeny.[13]
P. caryophila are parasitic inside the nucleus of P. biaurelia. As with other species of Holospora, they rely on the protist for amino acids, energy metabolic pathways including glycolysis and the citric acid cycle, as well as for nucleotides.[14] In contrast, Bealeia paramacronuclearis and Fokinia cryptica are cytoplasmic parasites. Bealeia paramacronuclearis are spherical or oval shaped measuring about 1.8 to 2.4 μm long and 0.4 to 0.5 μm broad. Inside the protist, they are arranged in clusters of about 7 to 8 cells in parallel lines. They mostly lie close to the macronucleus (the reason for the species name) and sometimes appear attached to the nuclear envelope. Fokinia cryptica are smaller with the diameter 0.35 to 0.40 μm. They are more randomly distributed in the cytoplasm.[4]
Distribution
Paramecium biaurelia is present in Europe, Asia, Australia, New Zealand, Tasmania, and North and South America.[15] It is most abundant in cold to moderate climates but does not occur in the tropics.[2]
References
- Sonneborn, T. M. (1975). "The Paramecium aurelia Complex of Fourteen Sibling Species". Transactions of the American Microscopical Society. 94 (2): 155–178. doi:10.2307/3224977. JSTOR 3224977.
- Tarcz, Sebastian; Sawka-Gądek, Natalia; Przyboś, Ewa (2018). "Worldwide sampling reveals low genetic variability in populations of the freshwater ciliate Paramecium biaurelia (P. aurelia species complex, Ciliophora, Protozoa)". Organisms Diversity & Evolution. 18 (1): 39–50. doi:10.1007/s13127-017-0357-z. S2CID 3863879.
- Barhey, K.; Gibson, I. (1984). "A study on the conditions for infection of Holospora caryophila, a macronuclear symbiont of Paramecium biaurelia". Micron and Microscopica Acta. 15 (4): 261–268. doi:10.1016/0739-6260(84)90040-6.
- Szokoli, Franziska; Castelli, Michele; Sabaneyeva, Elena; Schrallhammer, Martina; Krenek, Sascha; Doak, Thomas G.; Berendonk, Thomas U.; Petroni, Giulio (2016). Drake, H. L. (ed.). "Disentangling the Taxonomy of Rickettsiales and Description of Two Novel Symbionts ("Candidatus Bealeia paramacronuclearis" and "Candidatus Fokinia cryptica") Sharing the Cytoplasm of the Ciliate Protist Paramecium biaurelia". Applied and Environmental Microbiology. 82 (24): 7236–7247. Bibcode:2016ApEnM..82.7236S. doi:10.1128/AEM.02284-16. PMC 5118934. PMID 27742680.
- Beier, Cora L.; Horn, Matthias; Michel, Rolf; Schweikert, Michael; Görtz, Hans-Dieter; Wagner, Michael (2002). "The Genus Caedibacter Comprises Endosymbionts of Paramecium spp. Related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria)". Applied and Environmental Microbiology. 68 (12): 6043–6050. Bibcode:2002ApEnM..68.6043B. doi:10.1128/AEM.68.12.6043-6050.2002. PMC 134415. PMID 12450827.
- Bella, Chiara; Koehler, Lars; Grosser, Katrin; Berendonk, Thomas U.; Petroni, Giulio; Schrallhammer, Martina (2016). "Fitness Impact of Obligate Intranuclear Bacterial Symbionts Depends on Host Growth Phase". Frontiers in Microbiology. 7: 2084. doi:10.3389/fmicb.2016.02084. PMC 5177645. PMID 28066397.
- Krause, Martin; Bräucker, Richard; Hemmersbach, Ruth (2006). "Graviresponses of Paramecium biaurelia during parabolic flights". Protoplasma. 229 (2–4): 109–116. doi:10.1007/s00709-006-0207-x. PMID 17180491. S2CID 25716421.
- Hemmersbach, R.; Voormanns, R.; Hader, D. P. (1996). "Graviresponses in Paramecium biaurelia under different accelerations: studies on the ground and in space". The Journal of Experimental Biology. 199 (Pt 10): 2199–2205. doi:10.1242/jeb.199.10.2199. PMID 11541118.
- Hemmersbach, R.; Voormanns, R.; Bromeis, B.; Schmidt, N.; Rabien, H.; Ivanova, K. (1998). "Comparative studies of the graviresponses of paramecium and loxodes". Advances in Space Research. 21 (8–9): 1285–1289. Bibcode:1998AdSpR..21.1285H. doi:10.1016/S0273-1177(97)00400-6. PMID 11541383.
- Sonneborn, T. M. (1950). "Methods in the general biology and genetics of paramecium aurelia". Journal of Experimental Zoology. 113 (1): 87–147. doi:10.1002/jez.1401130106.
- Tarcz, Sebastian; Przyboś, Ewa; Surmacz, Marta (2013). "An assessment of haplotype variation in ribosomal and mitochondrial DNA fragments suggests incomplete lineage sorting in some species of the Paramecium aurelia complex (Ciliophora, Protozoa)". Molecular Phylogenetics and Evolution. 67 (1): 255–265. doi:10.1016/j.ympev.2013.01.016. PMID 23396203.
- Bella, Chiara; Koehler, Lars; Grosser, Katrin; Berendonk, Thomas U.; Petroni, Giulio; Schrallhammer, Martina (2016). "Fitness Impact of Obligate Intranuclear Bacterial Symbionts Depends on Host Growth Phase". Frontiers in Microbiology. 7: 2084. doi:10.3389/fmicb.2016.02084. PMC 5177645. PMID 28066397.
- Potekhin, Alexey; Schweikert, Michael; Nekrasova, Irina; Vitali, Valerio; Schwarzer, Sabine; Anikina, Arina; Kaltz, Oliver; Petroni, Giulio; Schrallhammer, Martina (2018). "Complex life cycle, broad host range and adaptation strategy of the intranuclear Paramecium symbiont Preeria caryophila comb. nov". FEMS Microbiology Ecology. 94 (7). doi:10.1093/femsec/fiy076. PMID 29718229. S2CID 13746190.
- Garushyants, Sofya K.; Beliavskaia, Alexandra Y.; Malko, Dmitry B.; Logacheva, Maria D.; Rautian, Maria S.; Gelfand, Mikhail S. (2018). "Comparative Genomic Analysis of Holospora spp., Intranuclear Symbionts of Paramecia". Frontiers in Microbiology. 9: 738. doi:10.3389/fmicb.2018.00738. PMC 5911502. PMID 29713316.
- Przyboś, Ewa; Surmacz, Marta (2010). "New, World-wide Data on the Distribution of Species of the Paramecium aurelia Complex (Ciliophora, Protozoa)". Folia Biologica. 58 (3): 185–188. doi:10.3409/fb58_3-4.185-188. PMID 20968184.