Pexacerfont
Pexacerfont (INN,[1] previously known as BMS-562,086) is a drug developed by Bristol-Myers Squibb which acts as a CRF1 antagonist.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
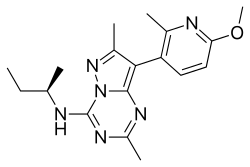
| Formula | C18H24N6O |
| Molar mass | 340.431 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Corticotropin-releasing factor (CRF), also known as corticotropin-releasing hormone, is an endogenous peptide hormone which is released in response to various triggers such as chronic stress. This then triggers the release of corticotropin (ACTH), another hormone which is involved in the physiological response to stress. Chronic release of CRF and ACTH is believed to be directly or indirectly involved in many of the harmful physiological effects of chronic stress, such as excessive glucocorticoid release, diabetes mellitus, osteoporosis, stomach ulcers, anxiety, depression, and development of high blood pressure and consequent cardiovascular problems.[2]
Pexacerfont is a recently developed CRF-1 antagonist which was in clinical trials for the treatment of anxiety disorders,[3] and has also been proposed to be useful for the treatment of depression and irritable bowel syndrome.
A recent multicenter, randomized, double-blind, placebo-controlled trial found that pexacerfont (100 mg/day) did not separate from placebo on the primary outcome measure (the mean change from baseline to end point in the Hamilton Anxiety Scale score).[4] These results suggest that blockade of CRF1 receptor may not be a feasible treatment for anxiety disorders in certain human populations.
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). RECOMMENDED International Nonproprietary Names: List 59" (PDF). World Health Organization. p. 60. Retrieved 16 July 2016.
- Zoumakis E, Rice KC, Gold PW, Chrousos GP (November 2006). "Potential uses of corticotropin-releasing hormone antagonists". Annals of the New York Academy of Sciences. 1083 (1): 239–51. Bibcode:2006NYASA1083..239Z. doi:10.1196/annals.1367.021. PMID 17148743. S2CID 7731338.
- Clinical trial number NCT00481325 for "Study of Pexacerfont (BMS-562086) in the Treatment of Outpatients With Generalized Anxiety Disorder" at ClinicalTrials.gov
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. (May 2010). "Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder". Depression and Anxiety. 27 (5): 417–25. doi:10.1002/da.20695. PMID 20455246. S2CID 5370614.