Pseudohypericin
Pseudohypericin is an aromatic polycyclic dione that is very closely related to hypericin. It is found most commonly in the St. John's wort family of plants, namely in Hypericum perforatum.[1] In preliminary studies in animal models, pseudohypericin has shown antiviral effects.[2][3] It may also contribute to the potential antidepressant effect of Hypericum perforatum extracts.[4]
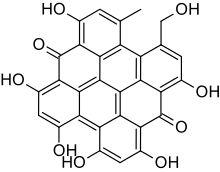 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,4,6,8,13-Hexahydroxy-10-(hydroxymethyl)-11-methylphenanthro[3,4,5,6-fghij]perylene-7,14-dione | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.111.993 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C30H16O9 |
| Molar mass | 520.449 g·mol−1 |
| log P | 4.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Kitanov, Gerassim M. (2001). "Hypericin and pseudohypericin in some Hypericum species". Biochemical Systematics and Ecology. 29 (2): 171–178. doi:10.1016/S0305-1978(00)00032-6. PMID 11106845.
- Meruelo, D.; Lavie, G.; Lavie, D. (1988). "Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin". Proceedings of the National Academy of Sciences. 85 (14): 5230–5234. Bibcode:1988PNAS...85.5230M. doi:10.1073/pnas.85.14.5230. PMC 281723. PMID 2839837.
- Hudson, J.B.; Lopez-Bazzocchi, I.; Towers, G.H.N. (1991). "Antiviral activities of hypericin". Antiviral Research. 15 (2): 101–112. doi:10.1016/0166-3542(91)90028-P. PMID 1650164.
- Butterweck, Veronika; Petereit, Frank; Winterhoff, Hilke; Nahrstedt, Adolf (1998). "Solubilized Hypericin and Pseudohypericin from Hypericum perforatum Exert Antidepressant Activity in the Forced Swimming Test3". Planta Medica. 64 (4): 291–294. doi:10.1055/s-2006-957437. PMID 9619107.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.