Q10 (temperature coefficient)
The Q10 temperature coefficient is a measure of temperature sensitivity based on the chemical reactions.
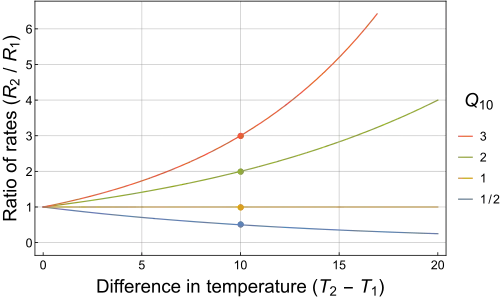
The Q10 is calculated as:
where;
- R is the rate
- T is the temperature in Celsius degrees or kelvin.
Rewriting this equation, the assumption behind Q10 is that the reaction rate R depends exponentially on temperature:
Q10 is a unitless quantity, as it is the factor by which a rate changes, and is a useful way to express the temperature dependence of a process.
For most biological systems, the Q10 value is ~ 2 to 3.[1]
In muscle performance
The temperature of a muscle has a significant effect on the velocity and power of the muscle contraction, with performance generally declining with decreasing temperatures and increasing with rising temperatures. The Q10 coefficient represents the degree of temperature dependence a muscle exhibits as measured by contraction rates.[2] A Q10 of 1.0 indicates thermal independence of a muscle whereas an increasing Q10 value indicates increasing thermal dependence. Values less than 1.0 indicate a negative or inverse thermal dependence, i.e., a decrease in muscle performance as temperature increases.[3]
Q10 values for biological processes vary with temperature. Decreasing muscle temperature results in a substantial decline of muscle performance such that a 10 degree Celsius temperature decrease results in at least a 50% decline in muscle performance.[4] Persons who have fallen into icy water may gradually lose the ability to swim or grasp safety lines due to this effect, although other effects such as atrial fibrillation are a more immediate cause of drowning deaths. At some minimum temperature biological systems do not function at all, but performance increases with rising temperature (Q10 of 2-4) to a maximum performance level and thermal independence (Q10 of 1.0-1.5). With continued increase in temperature, performance decreases rapidly (Q10 of 0.2-0.8) up to a maximum temperature at which all biological function again ceases.[5]
Within vertebrates, different skeletal muscle activity has correspondingly different thermal dependencies. The rate of muscle twitch contractions and relaxations are thermally dependent (Q10 of 2.0-2.5), whereas maximum contraction, e.g., tetanic contraction, is thermally independent.[6]
Muscles of some ectothermic species. e.g., sharks, show less thermal dependence at lower temperatures than endothermic species [4][7]
See also
- Arrhenius equation
- Arrhenius plot
- Isotonic (exercise physiology)
- Isometric exercise
- Skeletal striated muscle
- Tetanic contraction
References
- Reyes BA, Pendergast JS, Yamazaki S (February 2008). "Mammalian peripheral circadian oscillators are temperature compensated". Journal of Biological Rhythms. 23 (1): 95–8. doi:10.1177/0748730407311855. PMC 2365757. PMID 18258762.
- Mundim KC, Baraldi S, Machado HG, Vieira FM (2020-09-01). "Temperature coefficient (Q10) and its applications in biological systems: Beyond the Arrhenius theory". Ecological Modelling. 431: 109127. doi:10.1016/j.ecolmodel.2020.109127. ISSN 0304-3800.
- Bennett AF (August 1984). "Thermal dependence of muscle function". The American Journal of Physiology. 247 (2 Pt 2): R217-29. doi:10.1152/ajpregu.1984.247.2.R217. PMID 6380314.
- Deban SM, Lappin AK (April 2011). "Thermal effects on the dynamics and motor control of ballistic prey capture in toads: maintaining high performance at low temperature". The Journal of Experimental Biology. 214 (Pt 8): 1333–46. doi:10.1242/jeb.048405. PMID 21430211.
- Bennett AF (August 1990). "Thermal dependence of locomotor capacity". The American Journal of Physiology. 259 (2 Pt 2): R253-8. doi:10.1152/ajpregu.1990.259.2.R253. PMID 2201218.
- Bennett AF (March 1985). "Temperature and muscle". The Journal of Experimental Biology. 115: 333–44. PMID 3875678.
- Donley JM, Shadwick RE, Sepulveda CA, Syme DA (April 2007). "Thermal dependence of contractile properties of the aerobic locomotor muscle in the leopard shark and shortfin mako shark". The Journal of Experimental Biology. 210 (Pt 7): 1194–203. doi:10.1242/jeb.02730. PMID 17371918.