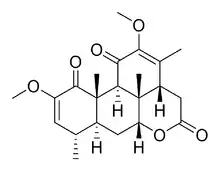
Quassin
Quassin is a white, bitter, crystalline substance that is the prototypical example of the family of quassinoids. It can be extracted from the quassia tree, from which it gets its name. It was first isolated in 1937 and its chemical structure was elucidated in 1961. It is one of the most bitter substances found in nature, with a bitter threshold of 0.08 ppm and it is 50 times more bitter than quinine.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2,12-Dimethoxypicrasa-2,12-diene-1,11,16-trione | |
| Preferred IUPAC name
(3aR,3a1S,6aR,7aS,8S,11aS,11bS)-2,10-Dimethoxy-3,3a1,8,11a-tetramethyl-3a,3a1,6a,7,7a,8,11a,11b-octahydrophenanthro[10,1-bc]pyran-1,5,11(4H)-trione | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.897 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H28O6 |
| Molar mass | 388.460 g·mol−1 |
| Appearance | White crystalline substance |
| Melting point | 200 to 222 °C (392 to 432 °F; 473 to 495 K) |
| Boiling point | 586 °C (1,087 °F; 859 K) |
Solubility in water |
Insoluble |
| Vapor pressure | 13 mmHg (@25 °C) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Extracts of the bitterwood tree (Quassia amara) containing quassin are used as additives in soft drinks.[1]
Although its skeleton possesses 20 carbon atoms, quassin is not a diterpene but rather a triterpene lactone, which derives from euphol by loss of 10 carbon atoms including C4.
References
- Scientific Committee on Food Opinion of the Scientific Committee on Food on quassin (expressed on 2 July 2002). Archived 19 May 2011 at the Wayback Machine SCF/CS/FLAV/FLAVOUR/29 Final
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.