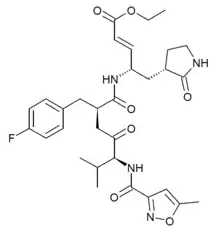
Rupintrivir
Rupintrivir (AG-7088, Rupinavir) is a peptidomimetic antiviral drug which acts as a 3C and 3CL protease inhibitor.[1][2][3] It was developed for the treatment of rhinoviruses,[4][5] and has subsequently been investigated for the treatment of other viral diseases including those caused by picornaviruses,[6][7] norovirus,[8] and coronaviruses, such as SARS and COVID-19.[9][10]
 | |
| Clinical data | |
|---|---|
| Trade names | Rupintrivir |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H39FN4O7 |
| Molar mass | 598.7 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, et al. (April 1999). "Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements". Journal of Medicinal Chemistry. 42 (7): 1213–24. doi:10.1021/jm9805384. PMID 10197965.
- Santos MM, Moreira R (October 2007). "Michael acceptors as cysteine protease inhibitors". Mini Reviews in Medicinal Chemistry. 7 (10): 1040–50. doi:10.2174/138955707782110105. PMID 17979807.
- Yuan S, Fan K, Chen Z, Sun Y, Hou H, Zhu L (February 2020). "Structure of the HRV-C 3C-Rupintrivir Complex Provides New Insights for Inhibitor Design". Virologica Sinica. 35 (4): 445–454. doi:10.1007/s12250-020-00196-4. PMC 7462945. PMID 32103448.
- Patick AK, Binford SL, Brothers MA, Jackson RL, Ford CE, Diem MD, et al. (October 1999). "In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease". Antimicrobial Agents and Chemotherapy. 43 (10): 2444–50. doi:10.1128/AAC.43.10.2444. PMC 89498. PMID 10508022.
- Jensen LM, Walker EJ, Jans DA, Ghildyal R (2015). "Proteases of human rhinovirus: role in infection". Rhinoviruses. Methods in Molecular Biology. Vol. 1221. pp. 129–41. doi:10.1007/978-1-4939-1571-2_10. ISBN 978-1-4939-1570-5. PMID 25261311.
- Barnard DL (2006). "Current status of anti-picornavirus therapies". Current Pharmaceutical Design. 12 (11): 1379–90. doi:10.2174/138161206776361129. PMID 16611122.
- De Palma AM, Vliegen I, De Clercq E, Neyts J (November 2008). "Selective inhibitors of picornavirus replication". Medicinal Research Reviews. 28 (6): 823–84. doi:10.1002/med.20125. PMID 18381747. S2CID 1575335.
- Rocha-Pereira J, Nascimento MS, Ma Q, Hilgenfeld R, Neyts J, Jochmans D (August 2014). "The enterovirus protease inhibitor rupintrivir exerts cross-genotypic anti-norovirus activity and clears cells from the norovirus replicon". Antimicrobial Agents and Chemotherapy. 58 (8): 4675–81. doi:10.1128/AAC.02546-13. PMC 4136040. PMID 24890597.
- Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R (June 2003). "Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs". Science. 300 (5626): 1763–7. Bibcode:2003Sci...300.1763A. doi:10.1126/science.1085658. PMID 12746549.
- Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. (2020). "Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases". ACS Central Science. 6 (3): 315–331. doi:10.1021/acscentsci.0c00272. PMC 7094090. PMID 32226821.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.