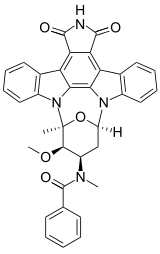
Stauprimide
Stauprimide is a semi-synthetic analog of the staurosporine family of indolocarbazoles. Stauprimide was first published in 1994 as part of an extensive structure-activity investigation to improve the selective inhibition of protein kinase C as a potential antitumor agent.[1] More recently, stauprimide has been shown to increase the efficiency of the directed differentiation of mouse and human embryonic stem cells in synergy with defined extracellular signaling cues. Stauprimide interacts with NME2 (PUF) transcription factor to down-regulate c-Myc expression, leading to differentiation of stem cells.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-[(12S,13R,14R,16R)-13-Methoxy-12-methyl-5,7-dioxo-6,7,13,14,15,16-hexahydro-5H,12H-17-oxa-11b,16a-diaza-12,16-methanocyclonona[jkl]cyclopenta[e]dibenzo[b,h]-as-indacen-14-yl]-N-methylbenzamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C35H28N4O5 |
| Molar mass | 584.632 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- Caravatti, Giorgio; Meyer, Thomas; Fredenhagen, Andreas; Trinks, Uwe; Mett, Helmut; Fabbro, Doriano (1994). "Inhibitory activity and selectivity of staurosporine derivatives towards protein kinase C". Bioorganic & Medicinal Chemistry Letters. 4 (3): 399–404. doi:10.1016/0960-894X(94)80004-9.
- Zhu, S; Wurdak, H; Wang, J; Lyssiotis, CA; Peters, EC; Cho, CY; Wu, X; Schultz, PG (2009). "A small molecule primes embryonic stem cells for differentiation". Cell Stem Cell. 4 (5): 416–26. doi:10.1016/j.stem.2009.04.001. PMID 19427291.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.