Talarozole
Talarozole (formerly R115866, planned trade name Rambazole) was an investigational drug for the treatment of acne, psoriasis and other keratinization disorders. Development has been discontinued.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth, topical |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
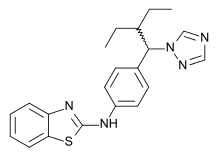
| Formula | C21H23N5S |
| Molar mass | 377.51 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Talarozole inhibits the metabolism of retinoic acid by blocking cytochrome P450 enzyme CYP26 isoenzymes (CYP26A1 and possibly also CYP26B1), retinoic acid hydroxylases.[2] Because of this mechanism, it is called a retinoic acid metabolism blocking agent (RAMBA).[2][3]
It has 750-fold higher potency than the earlier drug liarozole as well as greater selectivity, with more than 300-fold selectivity for inhibition of CYP26A1 over other steroid-metabolizing enzymes like CYP17A1 (17α-hydroxylase/17,20-lyase) and aromatase (CYP19A1).[2][4]
References
- "Talarozole - AdisInsight".
- Nelson CH, Buttrick BR, Isoherranen N (2013). "Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics". Curr Top Med Chem. 13 (12): 1402–28. doi:10.2174/1568026611313120004. PMC 4366427. PMID 23688132.
- Giltaire, S; Herphelin F; Frankart A; Hérin M; Stoppie P; Poumay Y (11 December 2008). "The CYP26 inhibitor R115866 potentiates the effects of all-trans retinoic acid on cultured human epidermal keratinocytes". Br J Dermatol. 160 (3): 505–13. doi:10.1111/j.1365-2133.2008.08960.x. PMID 19120344. S2CID 205258196.
- Gomaa MS, Lim AS, Lau SC, Watts AM, Illingworth NA, Bridgens CE, Veal GJ, Redfern CP, Brancale A, Armstrong JL, Simons C (2012). "Synthesis and CYP26A1 inhibitory activity of novel methyl 3-[4-(arylamino)phenyl]-3-(azole)-2,2-dimethylpropanoates". Bioorg. Med. Chem. 20 (20): 6080–8. doi:10.1016/j.bmc.2012.08.044. PMID 22989911.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.