Ammonoidea
Ammonoids are a group of extinct marine mollusc animals in the subclass Ammonoidea of the class Cephalopoda. These molluscs, commonly referred to as ammonites, are more closely related to living coleoids (i.e., octopuses, squid and cuttlefish) than they are to shelled nautiloids such as the living Nautilus species.[4] The earliest ammonites appeared during the Devonian, with the last species vanishing shortly after the Cretaceous–Paleogene extinction event, during the Danian epoch of the Paleocene.
| Ammonoids | |
|---|---|
 | |
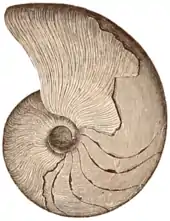
| Specimen of Pleuroceras solare, from the Lower Jurassic of Bavaria, Germany | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Cephalopoda |
| Subclass: | †Ammonoidea Zittel, 1884 |
| Orders | |
| |

Ammonites are excellent index fossils, and linking the rock layer in which a particular species or genus is found to specific geologic time periods is often possible. Their fossil shells usually take the form of planispirals, although some helically spiraled and nonspiraled forms (known as heteromorphs) have been found.
The name "ammonite", from which the scientific term is derived, was inspired by the spiral shape of their fossilized shells, which somewhat resemble tightly coiled rams' horns. Pliny the Elder (d. 79 AD near Pompeii) called fossils of these animals ammonis cornua ("horns of Ammon") because the Egyptian god Ammon (Amun) was typically depicted wearing rams' horns.[5] Often, the name of an ammonite genus ends in -ceras, which is from κέρας (kéras) meaning "horn".
Diagnostic characters
Ammonites (subclass Ammonoidea) can be distinguished by their septa, the dividing walls that separate the chambers in the phragmocone, by the nature of their sutures where the septa join the outer shell wall, and in general by their siphuncles.
Septa
Ammonoid septa characteristically have bulges and indentations and are to varying degrees convex when seen from the front, distinguishing them from nautiloid septa, which are typically simple concave, dish-shaped structures. The topology of the septa, especially around the rim, results in the various suture patterns found.[6]
Suture patterns

While nearly all nautiloids show gently curving sutures, the ammonoid suture line (the intersection of the septum with the outer shell) is variably folded, forming saddles ("peaks" that point towards the aperture) and lobes ("valleys" which point away from the aperture). The suture line has four main regions. The external or ventral region refers to sutures along the lower (outer) edge of the shell, where the left and right suture lines meet. The external saddle lies directly on the lower midline of the shell and is edged by external lobes. On suture diagrams the external saddle is supplied with an arrow which typically points towards the aperture. The lateral region involves the first saddle and lobe pair past the external region as the suture line extends up the side of the shell. Additional lobes developing towards the inner edge of a whorl are labelled umbilical lobes, which increase in number through ammonoid evolution as well as an individual ammonoid's development. Lobes and saddles which are so far towards the center of the whorl that they are covered up by succeeding whorls are labelled internal lobes and saddles. Three major types of suture patterns are found in the Ammonoidea:
- Goniatitic – numerous undivided lobes and saddles; typically 8 lobes around the conch. This pattern is characteristic of the Paleozoic ammonoids.
- Ceratitic – lobes have subdivided tips, giving them a saw-toothed appearance and rounded, undivided saddles. This suture pattern is characteristic of Triassic ammonoids and appears again in the Cretaceous "pseudoceratites".
- Ammonitic – lobes and saddles are much subdivided (fluted); subdivisions are usually rounded instead of saw-toothed. Ammonoids of this type are the most important species from a biostratigraphical point of view. This suture type is characteristic of Jurassic and Cretaceous ammonoids, but extends back all the way to the Permian.
 Goniatites plebeiformis showing Goniatitic suture
Goniatites plebeiformis showing Goniatitic suture Protrachyceras pseudoarchelonus showing Ceratitic suture
Protrachyceras pseudoarchelonus showing Ceratitic suture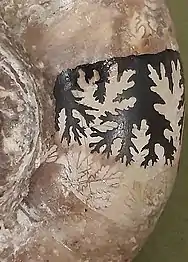 Lytoceras sutile showing Ammonitic suture
Lytoceras sutile showing Ammonitic suture
Siphuncle
The siphuncle in most ammonoids is a narrow tubular structure that runs along the shell's outer rim, known as the venter, connecting the chambers of the phragmocone to the body or living chamber. This distinguishes them from living nautiloides (Nautilus and Allonautilus) and typical Nautilida, in which the siphuncle runs through the center of each chamber. However the very earliest nautiloids from the Late Cambrian and Ordovician typically had ventral siphuncles like ammonites, although often proportionally larger and more internally structured. The word "siphuncle" comes from the New Latin siphunculus, meaning "little siphon".
Classification

Originating from within the bactritoid nautiloids, the ammonoid cephalopods first appeared in the Devonian (circa 409 million years ago (Mya)) and became extinct shortly after Cretaceous (66 Mya). The classification of ammonoids is based in part on the ornamentation and structure of the septa comprising their shells' gas chambers.
Orders and suborders


The Ammonoidea can be divided into six orders, listed here starting with the most primitive and going to the more derived:
- Agoniatitida, Lower Devonian – Middle Devonian
- Clymeniida, Upper Devonian
- Goniatitida, Middle Devonian – Upper Permian
- Prolecanitida, Upper Devonian – Upper Triassic
- Ceratitida, Upper Permian – Upper Triassic
- Ammonitida, Lower Jurassic – Lower Paleocene
In some classifications, these are left as suborders, included in only three orders: Goniatitida, Ceratitida and Ammonitida.
Taxonomy of the Treatise on Invertebrate Paleontology
The Treatise on Invertebrate Paleontology (Part L, 1957) divides the Ammonoidea, regarded simply as an order, into eight suborders, the Anarcestina, Clymeniina, Goniatitina and Prolecanitina from the Paleozoic; the Ceratitina from the Triassic; and the Ammonitina, Lytoceratina and Phylloceratina from the Jurassic and Cretaceous. In subsequent taxonomies, these are sometimes regarded as orders within the subclass Ammonoidea.
Life

Because ammonites and their close relatives are extinct, little is known about their way of life. Their soft body parts are very rarely preserved in any detail. Nonetheless, much has been worked out by examining ammonoid shells and by using models of these shells in water tanks.
Many ammonoids probably lived in the open water of ancient seas, rather than at the sea bottom, because their fossils are often found in rocks laid down under conditions where no bottom-dwelling life is found. Many of them (such as Oxynoticeras) are thought to have been good swimmers, with flattened, discus-shaped, streamlined shells, although some ammonoids were less effective swimmers and were likely to have been slow-swimming bottom-dwellers. Synchrotron analysis of an aptychophoran ammonite revealed remains of isopod and mollusc larvae in its buccal cavity, indicating at least this kind of ammonite fed on plankton.[7] They may have avoided predation by squirting ink, much like modern cephalopods; ink is occasionally preserved in fossil specimens.[8]
The soft body of the creature occupied the largest segments of the shell at the end of the coil. The smaller earlier segments were walled off and the animal could maintain its buoyancy by filling them with gas. Thus, the smaller sections of the coil would have floated above the larger sections.[9]
Many ammonite shells have been found with round holes once interpreted as a result of limpets attaching themselves to the shells. However, the triangular formation of the holes, their size and shape, and their presence on both sides of the shells, corresponding to the upper and lower jaws, is more likely evidence of the bite of a medium-sized mosasaur preying upon ammonites.
Some ammonites appear to have lived in cold seeps and even reproduced there.[10]
Shell anatomy and diversity


Basic shell anatomy



The chambered part of the ammonite shell is called a phragmocone. It contains a series of progressively larger chambers, called camerae (sing. camera) that are divided by thin walls called septa (sing. septum). Only the last and largest chamber, the body chamber, was occupied by the living animal at any given moment. As it grew, it added newer and larger chambers to the open end of the coil. Where the outer whorl of an ammonite shell largely covers the preceding whorls, the specimen is said to be involute (e.g., Anahoplites). Where it does not cover those preceding, the specimen is said to be evolute (e.g., Dactylioceras).
A thin living tube called a siphuncle passed through the septa, extending from the ammonite's body into the empty shell chambers. Through a hyperosmotic active transport process, the ammonite emptied water out of these shell chambers. This enabled it to control the buoyancy of the shell and thereby rise or descend in the water column.
A primary difference between ammonites and nautiloids is the siphuncle of ammonites (excepting Clymeniina) runs along the ventral periphery of the septa and camerae (i.e., the inner surface of the outer axis of the shell), while the siphuncle of nautiloids runs more or less through the center of the septa and camerae.
Sexual dimorphism

One feature found in shells of the modern Nautilus is the variation in the shape and size of the shell according to the sex of the animal, the shell of the male being slightly smaller and wider than that of the female. This sexual dimorphism is thought to be an explanation for the variation in size of certain ammonite shells of the same species, the larger shell (the macroconch) being female, and the smaller shell (the microconch) being male. This is thought to be because the female required a larger body size for egg production. A good example of this sexual variation is found in Bifericeras from the early part of the Jurassic period of Europe.
Only recently has sexual variation in the shells of ammonites been recognized. The macroconch and microconch of one species were often previously mistaken for two closely related but different species occurring in the same rocks. However, because the dimorphic sizes are so consistently found together, they are more likely an example of sexual dimorphism within the same species.
Whorl width in the body chamber of many groups of ammonites, as expressed by the width:diameter ratio, is another sign of dimorphism. This character has been used to separate "male" (Largiventer conch "L") from "female" (Leviventer conch "l").[11]
Variations in shape

The majority of ammonite species feature planispiral, flat-coiled shells, but other species feature nearly straight (as in baculites) shells. Still other species' shells are coiled helically, similar in appearance to some gastropods (e.g., Turrilites and Bostrychoceras). Some species' shells are even initially uncoiled, then partially coiled, and finally straight at maturity (as in Australiceras). These partially uncoiled and totally uncoiled forms began to diversify mainly during the early part of the Cretaceous and are known as heteromorphs.
Perhaps the most extreme and bizarre-looking example of a heteromorph is Nipponites, which appears to be a tangle of irregular whorls lacking any obvious symmetric coiling. Upon closer inspection, though, the shell proves to be a three-dimensional network of connected "U" shapes. Nipponites occurs in rocks of the upper part of the Cretaceous in Japan and the United States.
Ammonites vary greatly in the ornamentation (surface relief) of their shells. Some may be smooth and relatively featureless, except for growth lines, and resemble that of the modern Nautilus. In others, various patterns of spiral ridges and ribs or even spines are shown. This type of ornamentation of the shell is especially evident in the later ammonites of the Cretaceous.
Aptychus

Some ammonites have been found in association with a single horny plate or a pair of calcitic plates. In the past, these plates were assumed to serve in closing the opening of the shell in much the same way as an operculum, but more recently they are postulated to have been a jaw apparatus.[12][13][14][15]
The plates are collectively termed the aptychus or aptychi in the case of a pair of plates, and anaptychus in the case of a single plate. The paired aptychi were symmetric to one another and equal in size and appearance.
Anaptychi are relatively rare as fossils. They are found representing ammonites from the Devonian period through those of the Cretaceous period.
Calcified aptychi only occur in ammonites from the Mesozoic era. They are almost always found detached from the shell, and are only very rarely preserved in place. Still, sufficient numbers have been found closing the apertures of fossil ammonite shells as to leave no doubt as to their identity as part of the anatomy of an ammonite.
Large numbers of detached aptychi occur in certain beds of rock (such as those from the Mesozoic in the Alps). These rocks are usually accumulated at great depths. The modern Nautilus lacks any calcitic plate for closing its shell, and only one extinct nautiloid genus is known to have borne anything similar. Nautilus does, however, have a leathery head shield (the hood) which it uses to cover the opening when it retreats inside.
There are many forms of aptychus, varying in shape and the sculpture of the inner and outer surfaces, but because they are so rarely found in position within the shell of the ammonite it is often unclear to which species of ammonite one kind of aptychus belongs. A number of aptychi have been given their own genus and even species names independent of their unknown owners' genus and species, pending future discovery of verified occurrences within ammonite shells.
Soft-part anatomy
Although ammonites do occur in exceptional lagerstatten such as the Solnhofen Limestone, their soft-part record is surprisingly bleak. Beyond a tentative ink sac and possible digestive organs, no soft parts were known until 2021.[16][17] They likely bore a radula and beak, a marginal siphuncle and ten arms.[18] They operated by direct development with sexual reproduction, were carnivorous, and had a crop for food storage. They are unlikely to have dwelt in fresh or brackish water.[18] Many ammonites were likely filter feeders, so adaptations associated with this lifestyle like sieves probably occurred.[7]
A 2021 study found ammonite specimens with preserved hook-like suckers, providing a general shape to ammonite tentacles.[19] A contemporary study found an ammonite isolated body, offering for the first time a glimpse into these animals' organs.[17]
Size

The smallest ammonoid was Maximites from the Upper Carboniferous. Adult specimens reached only 10 mm (0.39 in) in shell diameter.[20] Few of the ammonites occurring in the lower and middle part of the Jurassic period reached a size exceeding 23 cm (9.1 in) in diameter. Much larger forms are found in the later rocks of the upper part of the Jurassic and the lower part of the Cretaceous, such as Titanites from the Portland Stone of Jurassic of southern England, which is often 53 cm (1.74 ft) in diameter, and Parapuzosia seppenradensis of the Cretaceous period of Germany, which is one of the largest-known ammonites, sometimes reaching 2 m (6.6 ft) in diameter. The largest-documented North American ammonite is Parapuzosia bradyi from the Cretaceous, with specimens measuring 137 cm (4.5 ft) in diameter.

Distribution

Starting from the mid-Devonian, ammonoids were extremely abundant, especially as ammonites during the Mesozoic era. Many genera evolved and ran their course quickly, becoming extinct in a few million years. Due to their rapid evolution and widespread distribution, ammonoids are used by geologists and paleontologists for biostratigraphy. They are excellent index fossils, and it is often possible to link the rock layer in which they are found to specific geologic time periods.
Due to their free-swimming and/or free-floating habits, ammonites often happened to live directly above seafloor waters so poor in oxygen as to prevent the establishment of animal life on the seafloor. When upon death the ammonites fell to this seafloor and were gradually buried in accumulating sediment, bacterial decomposition of these corpses often tipped the delicate balance of local redox conditions sufficiently to lower the local solubility of minerals dissolved in the seawater, notably phosphates and carbonates. The resulting spontaneous concentric precipitation of minerals around a fossil, a concretion, is responsible for the outstanding preservation of many ammonite fossils.
When ammonites are found in clays, their original mother-of-pearl coating is often preserved. This type of preservation is found in ammonites such as Hoplites from the Cretaceous Gault clay of Folkestone in Kent, England.
The Cretaceous Pierre Shale formation of the United States and Canada is well known for the abundant ammonite fauna it yields, including Baculites, Placenticeras, Scaphites, Hoploscaphites and Jeletzkytes, as well as many uncoiled forms. Many of these also have much or all of the original shell, as well as the complete body chamber, still intact. Many Pierre Shale ammonites, and indeed many ammonites throughout earth history, are found inside concretions.

Other fossils, such as many found in Madagascar and Alberta, Canada display iridescence. These iridescent ammonites are often of gem quality (ammolite) when polished. In no case would this iridescence have been visible during the animal's life; additional shell layers covered it.
The majority of ammonoid specimens, especially those of the Paleozoic era, are preserved only as internal molds; the outer shell (composed of aragonite)[21] has been lost during the fossilization process. Only in these internal-mould specimens can the suture lines be observed; in life, the sutures would have been hidden by the outer shell.
The ammonoids as a group continued through several major extinction events, although often only a few species survived. Each time, however, this handful of species diversified into a multitude of forms. Ammonite fossils became less abundant during the latter part of the Mesozoic, and although they seemingly survived the Cretaceous–Paleogene extinction event, all known Paleocene ammonite lineages are restricted to the Paleocene epoch (65–61 Ma).[22][23]
Evolutionary history
Goniatites, which were a dominant component of Early and Middle Permian faunas, became rare in the Late Permian, and no goniatite is thought to have crossed into the Triassic.[24]
Ceratitida originated during the Middle Permian, likely from the Daraelitidae, and radiated in the Late Permian. In the aftermath of the Permian–Triassic extinction event, Ceratitids represent the dominant group of Triassic ammonites.[24]
Ammonites were devastated by the end-Triassic extinction, with only a handful of genera belonging to the family Psiloceratidae of the suborder Phylloceratina surviving and becoming ancestral to all later Jurassic and Cretaceous ammonites. Ammonites explosively diversified during the Early Jurassic, with the orders Psiloceratina, Ammonitina, Lytoceratina, Haploceratina, Perisphinctina and Ancyloceratina all appearing during the Jurassic.[25]
Heteromorph ammonites (ammonites with open or non-spiral coiling) of the order Ancyloceratina became common during the Cretaceous period.[26]

The extinction of the ammonites, along with other marine animals and non-avian dinosaurs, has been attributed to the K–Pg extinction event, marking the end of the Cretaceous Period.
Eight or so species from only two families made it almost to the end of the Cretaceous, the order having gone through a more or less steady decline since the middle of the period. Six other families made it well into the upper Maastrichtian (uppermost stage of the Cretaceous), but were extinct well before the end. All told, 11 families entered the Maastrichtian, a decline from the 19 families known from the Cenomanian in the middle of the Cretaceous.
One reason given for their demise is the Cretaceous ammonites, being closely related to coleoids, had a similar reproductive strategy in which huge numbers of eggs were laid in a single batch at the end of the lifespan. These, along with juvenile ammonites, are thought to have been part of the plankton at the surface of the ocean, where they were killed off by the effects of an impact. Nautiloids, exemplified by modern nautiluses, are conversely thought to have had a reproductive strategy in which eggs were laid in smaller batches many times during the lifespan, and on the sea floor well away from any direct effects of such a bolide strike, and thus survived.[27]
Many ammonite species were filter feeders, so they might have been particularly susceptible to marine faunal turnovers and climatic change.[7]
There have been reliable reports of ammonite fossils from the early Paleocene. The main fossil finds have been concentrated in Denmark, the Netherlands, and the United States, with an additional possible record in Turkmenistan. However, the current fossil evidence is limited to a maximum of 200,000 years after the K/Pg boundary.[22][23][28][29]
Cultural significance
In medieval Europe, fossilised ammonites were thought to be petrified coiled snakes, and were called "snakestones" or, more commonly in medieval England, "serpentstones". They were considered to be evidence for the actions of saints, such as Hilda of Whitby, a myth referenced in Sir Walter Scott's Marmion,[30] and Saint Patrick, and were held to have healing or oracular powers. Traders would occasionally carve the head of a snake onto the empty, wide end of the ammonite fossil, and then sell them as petrified snakes. In other cases, the snake's head would be simply painted on.[31][32]
Ammonites from the Gandaki River in Nepal are known as saligrams, and are believed by Hindus to be a concrete manifestation of Vishnu.[33]
See also
- Ammolite – a gemstone formed from fossil ammonite shells
- Belemnoidea
- Coleoidea
- Geologic time scale
- Nautiloid
References
- "Map and table describing fossil collections and related samples in the Ketchikan and Prince Rupert quadrangles, southeastern Alaska". United States Geological Survey Open-File Report. doi:10.3133/ofr821088.
{{cite journal}}: Cite journal requires|journal=(help) - "Ammonoidea (ammonite)". PBDB.org.
- T, Hansen (1987). "Sedimentology and extinction patterns across the Cretaceous-Tertiary boundary interval in east Texas". Cretaceous Research. 8 (3). doi:10.1016/0195-6671(87)90023-1.
- Klug, Christian; Kröger, Björn; Vinther, Jakob; Fuchs, Dirk (August 2015). "Ancestry, Origin and Early Evolution of Ammonoids". In Christian Klug; Dieter Korn; Kenneth De Baets; Isabelle Kruta; Royal H. Mapes (eds.). Ammonoid Paleobiology: From macroevolution to paleogeography. Topics in Geobiology 44. Vol. 44. Springer. pp. 3–24. doi:10.1007/978-94-017-9633-0_1. ISBN 978-94-017-9632-3.
- NH 37.40.167
- "The Cephalopoda". ucmp.berkeley.edu. Retrieved September 24, 2019.
- Kruta, Isabelle; Landman, Neil; Rouget, Isabelle; Cecca, Fabrizio; Tafforeau, Paul (Jan 2011). "The Role of Ammonites in the Mesozoic Marine Food Web Revealed by Jaw Preservation". Science. 331 (6013): 70–72. Bibcode:2011Sci...331...70K. doi:10.1126/science.1198793. PMID 21212354. S2CID 206530342.
- Doguzhaeva, Larisa A.; Royal H. Mapes; Herbert Summesberger; Harry Mutvei (2007). "The Preservation of Body Tissues, Shell, and Mandibles in the Ceratitid Ammonoid Austrotrachyceras (Late Triassic), Austria". In N. H. Landman; et al. (eds.). Cephalopods Present and Past: New Insights and Fresh Perspectives. Dordrecht: Springer. pp. 221–238. doi:10.1007/978-1-4020-6806-5_11. ISBN 978-1-4020-6806-5.
- "Introduction to Ammonoidea". The Geology of Portsdown Hill. Archived from the original on 2 May 2007. Retrieved 2007-04-26.
- Rowe, Alison J.; Landman, Neil H.; Cochran, J. Kirk; Witts, James D.; Garb, Matthew P. (26 March 2020). "Late Cretaceous Methane Seeps as Habitats for Newly Hatched Ammonites". PALAIOS. 35 (3): 151–163. Bibcode:2020Palai..35..151R. doi:10.2110/palo.2019.105. S2CID 214718487.
- Sarti, Carlo (1999). "Whorl Width in the Body Chamber of Ammonites as a Sign of Dimorphism". Advancing Research on Living and Fossil Cephalopods. pp. 315–332. doi:10.1007/978-1-4615-4837-9_23. ISBN 978-1-4613-7193-9.
- Morton, N (1981). "Aptychi: the myth of the ammonite operculum". Lethaia. 14 (1): 57–61. doi:10.1111/j.1502-3931.1981.tb01074.x.
- Morton, N.; Nixon, M. (1987). "Size and function of ammonite aptychi in comparison with buccal masses of modem cephalopods". Lethaia. 20 (3): 231–238. doi:10.1111/j.1502-3931.1987.tb02043.x.
- Lehmann, U.; Kulicki, C. (1990). "Double function of aptychi (Ammonoidea) as jaw elements and opercula". Lethaia. 23 (4): 325–331. doi:10.1111/j.1502-3931.1990.tb01365.x.
- Seilacher, A (1993). "Ammonite aptychi; how to transform a jaw into an operculum?". American Journal of Science. 293: 20–32. Bibcode:1993AmJS..293...20S. doi:10.2475/ajs.293.A.20.
- Wippich, M. G. E.; Lehmann, J. (2004). "Allocrioceras from the Cenomanian (mid-Cretaceous) of the Lebanon and its bearing on the palaeobiological interpretation of heteromorphic ammonites". Palaeontology. 47 (5): 1093–1107. doi:10.1111/j.0031-0239.2004.00408.x.
- Klug, Christian; Schweigert, Günter; Tischlinger, Helmut; Pochmann, Helmut (December 2021). "Failed prey or peculiar necrolysis? Isolated ammonite soft body from the Late Jurassic of Eichstätt (Germany) with complete digestive tract and male reproductive organs". Swiss Journal of Palaeontology. 140 (1): 3. doi:10.1186/s13358-020-00215-7. PMC 7813712. PMID 33505352.
- Landman, Neil H; Tanabe, Kazushige; Davis, Richard Arnold (1996). Ammonoid paleobiology. ISBN 978-0-306-45222-2.
- Smith, C. P. A.; Landman, N. H.; Bardin, J.; Kruta, I. (4 June 2021). "New evidence from exceptionally "well-preserved" specimens sheds light on the structure of the ammonite brachial crown". Scientific Reports. 11 (1): 11862. Bibcode:2021NatSR..1111862S. doi:10.1038/s41598-021-89998-4. PMC 8178333. PMID 34088905.
- Nishiguchi, M.K., R. Mapes (2008). "Cephalopoda" (PDF). University of California Press. pp. 163–199.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Buchardt, B.; Weiner, S. (1981). "Diagenesis of aragonite from Upper Cretaceous ammonites: a geochemical case-study". Sedimentology. 28 (3): 423–438. Bibcode:1981Sedim..28..423B. doi:10.1111/j.1365-3091.1981.tb01691.x.
- Landman, Neil H.; Goolaerts, Stijn; Jagt, John W.M.; Jagt-Yazykova, Elena A.; Machalski, Marcin (2015), Klug, Christian; Korn, Dieter; De Baets, Kenneth; Kruta, Isabelle (eds.), "Ammonites on the Brink of Extinction: Diversity, Abundance, and Ecology of the Order Ammonoidea at the Cretaceous/Paleogene (K/Pg) Boundary", Ammonoid Paleobiology: From macroevolution to paleogeography, Dordrecht: Springer Netherlands, vol. 44, pp. 497–553, doi:10.1007/978-94-017-9633-0_19, ISBN 978-94-017-9632-3, retrieved 2021-10-26
- Machalski, Marcin; Heinberg, Claus (2005-12-01). "Evidence for ammonite survival into the Danian (Paleogene) from the Cerithium Limestone at Stevns Klint, Denmark". Bulletin of the Geological Society of Denmark. 52: 2005–12. doi:10.37570/bgsd-2005-52-08.
- McGowan, Alistair J.; Smith, Andrew B. (May 2007). "Ammonoids Across the Permian/Triassic Boundary: A Cladistic Perspective". Palaeontology. 50 (3): 573–590. doi:10.1111/j.1475-4983.2007.00653.x.
- Page, Kevin N. (January 2008). "The evolution and geography of Jurassic ammonoids". Proceedings of the Geologists' Association. 119 (1): 35–57. doi:10.1016/S0016-7878(08)80257-X.
- Hoffmann, René; Slattery, Joshua S.; Kruta, Isabelle; Linzmeier, Benjamin J.; Lemanis, Robert E.; Mironenko, Aleksandr; Goolaerts, Stijn; De Baets, Kenneth; Peterman, David J.; Klug, Christian (April 2021). "Recent advances in heteromorph ammonoid palaeobiology". Biological Reviews. 96 (2): 576–610. doi:10.1111/brv.12669. PMID 33438316. S2CID 231593832.
- Ward, Peter (1996). "20- Ammonoid Extinction". Ammonoid Paleobiology. Topics in Geobiology. Vol. 13. Springer. pp. 815–823. doi:10.1007/978-1-4757-9153-2_20. ISBN 978-1-4757-9155-6.
- Staaf, Danna (2017). Squid Empire: The Rise and Fall of the Cephalopods. University Press of New England. ISBN 978-1-61168-923-5.
- Machalski, Marcin; Heinberg, Claus (2005-12-01). "Evidence for ammonite survival into the Danian (Paleogene) from the Cerithium Limestone at Stevns Klint, Denmark". Bulletin of the Geological Society of Denmark. 52: 2005–12. doi:10.37570/bgsd-2005-52-08.
- Lovett, Edward (September 1905). "The Whitby Snake-Ammonite Myth". Folk-Lore. 16 (3): 333–4. doi:10.1080/0015587x.1905.9719966.
- Cadbury, D. The Dinosaur Hunters. (Fourth Estate, 2000) (ISBN 1-85702-963-1), p.7
- "Folklore 16(1905)333".
- "Fossils: myths, mystery, and magic". The Independent. 2007-02-12. Archived from the original on 2007-11-11. Retrieved 2010-04-23.
Further reading
- Larson, Neal L. (1997). Ammonites and the Other Cephalopods of the Pierre Seaway: Identification Guide. Geoscience Press. ISBN 978-0-945005-25-4.
- Lehmann, Ulrich (1981). The Ammonites: Their life and their world. Cambridge University Press. ISBN 978-0-521-23627-0.
- Monks, Neale; Palmer, Philip (2002). Ammonites. Smithsonian Institution Press. ISBN 978-1-58834-024-5.
- Walker, Cyril and Ward, David. Fossils. Dorling, Kindersley Limited, London, 2002.
- A Broad Brush History of the Cephalopoda by Dr. Neale Monks, from The Cephalopod Page.
- Ammonite maturity, pathology and old age By Dr. Neale Monks, from The Cephalopod Page. Essay about the life span of Ammonites.
- Cretaceous Fossils Taxonomic Index for Order Ammonoitida
- Deeply Buried Sediments Tell Story of Sudden Mass Extinction
External links
Ammonoidea
(Ammonites).
- Descriptions and pictures of ammonite fossils
- goniat.org, a palaezoic ammonoid database
- paleozoic.org: gallery of ammonite photographs
- photos of ammonites at Lyme Regis, UK
- TaxonConcept's data on cretaceous ammonites
- The ammonites of Peacehaven - photos of giant cretaceous ammonites in Southern England
- tonmo.com: The octopus news magazine online, Cephalopod fossil articles.
- William R. Wahl * Mosasaur Bite Marks on an Ammonite. Preservation of an Aborted Attack?
- Mosasaur diet