Beta-glucan
Beta-glucans, β-glucans comprise a group of β-D-glucose polysaccharides (glucans) naturally occurring in the cell walls of cereals, bacteria, and fungi, with significantly differing physicochemical properties dependent on source. Typically, β-glucans form a linear backbone with 1–3 β-glycosidic bonds but vary with respect to molecular mass, solubility, viscosity, branching structure, and gelation properties, causing diverse physiological effects in animals.
At dietary intake levels of at least 3 g per day, oat fiber β-glucan decreases blood levels of LDL cholesterol and so may reduce the risk of cardiovascular diseases.[1] β-glucans are natural gums and are used as texturing agents in various nutraceutical and cosmetic products, and as soluble fiber supplements.
History
Cereal and fungal products have been used for centuries for medicinal and cosmetic purposes; however, the specific role of β-glucan was not explored until the 20th century. β-glucans were first discovered in lichens, and shortly thereafter in barley. A particular interest in oat β-glucan arose after a cholesterol lowering effect from oat bran reported in 1981.[2]
In 1997, the FDA approved of a claim that intake of at least 3.0 g of β-glucan from oats per day decreased absorption of dietary cholesterol and reduced the risk of coronary heart disease. The approved health claim was later amended to include these sources of β-glucan: rolled oats (oatmeal), oat bran, whole oat flour, oatrim (the soluble fraction of alpha-amylase hydrolyzed oat bran or whole oat flour), whole grain barley and barley beta-fiber. An example of an allowed label claim: "Soluble fiber from foods such as oatmeal, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease. A serving of oatmeal supplies 0.75 grams of the 3.0 g of β-glucan soluble fiber necessary per day to have this effect." The claim language is in the Federal Register 21 CFR 101.81 Health Claims: "Soluble fiber from certain foods and risk of coronary heart disease (CHD)".[3]
Structure
Glucans are arranged in six-sided D-glucose rings connected linearly at varying carbon positions depending on the source, although most commonly β-glucans include a 1-3 glycosidic link in their backbone. Although technically β-glucans are chains of D-glucose polysaccharides linked by β-type glycosidic bonds, by convention not all β-D-glucose polysaccharides are categorized as β-glucans.[4] Cellulose is not conventionally considered a β-glucan, as it is insoluble and does not exhibit the same physicochemical properties as other cereal or yeast β-glucans.[5]
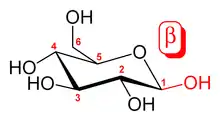
Some β-glucan molecules have branching glucose side-chains attached to other positions on the main D-glucose chain, which branch off the β-glucan backbone. In addition, these side-chains can be attached to other types of molecules, like proteins, as in polysaccharide-K.
The most common forms of β-glucans are those comprising D-glucose units with β-1,3 links. Yeast and fungal β-glucans contain 1-6 side branches, while cereal β-glucans contain both β-1,3 and β-1,4 backbone bonds. The frequency, location, and length of the side-chains may play a role in immunomodulation. Differences in molecular weight, shape, and structure of β-glucans dictate the differences in biological activity.[6][7]
In general, β-1,3 linkages are created by 1,3-Beta-glucan synthase, and β-1,4 linkages are created by cellulose synthase. The process leading to β-1,6 linkages is poorly understood: although genes important in the process have been identified, not much is known about what each of them do.[8]
| Source (Example) | Backbone | Branching | Solubility in Water |
|---|---|---|---|
| Bacteria (Curdlan) |  |
None | Insoluble[9] |
| Fungus |  |
Short β-1,6 branching | Insoluble[10] |
| Yeast |  |
Long β-1,6 branching | Insoluble[7] |
| Cereal (Oat beta-glucan) |  |
None | Soluble[6] |
β-glucan types
β-glucans form a natural component of the cell walls of bacteria, fungi, yeast, and cereals such as oat and barley. Each type of beta-glucan comprises a different molecular backbone, level of branching, and molecular weight which affects its solubility and physiological impact. One of the most common sources of β(1,3)D-glucan for supplement use is derived from the cell wall of baker's yeast (Saccharomyces cerevisiae). β-glucans found in the cell walls of yeast contain a 1,3 carbon backbone with elongated 1,6 carbon branches.[11] Other sources include seaweed,[12] and various mushrooms, such as lingzhi, shiitake, chaga, and maitake, which are under preliminary research for their potential immune effects.[13]
Fermentable fiber
In the diet, β-glucans are a source of soluble, fermentable fiber – also called prebiotic fiber – which provides a substrate for microbiota within the large intestine, increasing fecal bulk and producing short-chain fatty acids as byproducts with wide-ranging physiological activities.[14] This fermentation impacts the expression of many genes within the large intestine,[15] which further affects digestive function and cholesterol and glucose metabolism, as well as the immune system and other systemic functions.[14][16]
.jpg.webp)
Cereal
Cereal β-glucans from oat, barley, wheat, and rye have been studied for their effects on cholesterol levels in people with normal cholesterol levels and in those with hypercholesterolemia.[1] Intake of oat β-glucan at daily amounts of at least 3 grams lowers total and low-density lipoprotein cholesterol levels by 5 to 10% in people with normal or elevated blood cholesterol levels.[17]
Oats and barley differ in the ratio of trimer and tetramer 1-4 linkages. Barley has more 1-4 linkages with a degree of polymerization higher than 4. However, the majority of barley blocks remain trimers and tetramers. In oats, β-glucan is found mainly in the endosperm of the oat kernel, especially in the outer layers of that endosperm.[6]
β-glucan absorption
Enterocytes facilitate the transportation of β(1,3)-glucans and similar compounds across the intestinal cell wall into the lymph, where they begin to interact with macrophages to activate immune function.[18] Radiolabeled studies have verified that both small and large fragments of β-glucans are found in the serum, which indicates that they are absorbed from the intestinal tract.[19] M cells within the Peyer's patches physically transport the insoluble whole glucan particles into the gut-associated lymphoid tissue.[20]
(1,3)-β-D-glucan medical application
An assay to detect the presence of (1,3)-β-D-glucan in blood is marketed as a means of identifying invasive or disseminated fungal infections.[21][22][23] This test should be interpreted within the broader clinical context, however, as a positive test does not render a diagnosis, and a negative test does not rule out infection. False positives may occur because of fungal contaminants in the antibiotics amoxicillin-clavulanate,[24] and piperacillin/tazobactam. False positives can also occur with contamination of clinical specimens with the bacteria Streptococcus pneumoniae, Pseudomonas aeruginosa, and Alcaligenes faecalis, which also produce (1→3)β-D-glucan.[25] This test can aid in the detection of Aspergillus, Candida, and Pneumocystis jirovecii.[26][27][28] This test cannot be used to detect Mucor or Rhizopus, the fungi responsible for mucormycosis, as they do not produce (1,3)-beta-D-glucan.[29]
See also
- Prebiotic (nutrition)
- Resistant starch
- Xylooligosaccharides
References
- Ho, H. V; Sievenpiper, J. L; Zurbau, A; Blanco Mejia, S; Jovanovski, E; Au-Yeung, F; Jenkins, A. L; Vuksan, V (2016). "The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials". British Journal of Nutrition. 116 (8): 1369–1382. doi:10.1017/S000711451600341X. PMID 27724985.
- Kirby RW, Anderson JW, Sieling B, Rees ED, Chen WJ, Miller RE, Kay RM (1981). "Oat-bran intake selectively lowers serum low-density lipoprotein cholesterol concentrations of hypercholesterolemic men". Am. J. Clin. Nutr. 34 (5): 824–9. doi:10.1093/ajcn/34.5.824. PMID 6263072.
- https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_181 21 CFR 101.81 Health Claims: Soluble fiber from certain foods and risk of coronary heart disease (CHD)
- Zeković, Djordje B. (10 October 2008). "Natural and Modified (1→3)-β-D-Glucans in Health Promotion and Disease Alleviation". Critical Reviews in Biotechnology. 25 (4): 205–230. doi:10.1080/07388550500376166. PMID 16419618. S2CID 86109922.
- Sikora, Per (14 June 2012). "Identification of high b-glucan oat lines and localization and chemical characterization of their seed kernel b-glucans". Food Chemistry. 137 (1–4): 83–91. doi:10.1016/j.foodchem.2012.10.007. PMID 23199994.
- Chu, YiFang (2014). Oats Nutrition and Technology. Barrington, Illinois: Wiley Blackwell. ISBN 978-1-118-35411-7.
- Volman, Julia J (20 November 2007). "Dietary modulation of immune function by β-glucans". Physiology & Behavior. 94 (2): 276–284. doi:10.1016/j.physbeh.2007.11.045. PMID 18222501. S2CID 24758421.
- Ruiz-Herrera J, Ortiz-Castellanos L (May 2010). "Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi". FEMS Yeast Research. 10 (3): 225–43. doi:10.1111/j.1567-1364.2009.00589.x. PMID 19891730.
- Mcintosh, M (19 October 2004). "Curdlan and other bacterial (1→3)-β-D-glucans". Applied Microbiology and Biotechnology. 68 (2): 163–173. doi:10.1007/s00253-005-1959-5. PMID 15818477. S2CID 13123359.
- Han, Man Deuk (March 2008). "Solubilization of water-insoluble β-glucan isolated from Ganoderma lucidum". Journal of Environmental Biology.
- Manners, David J. (2 February 1973). "The Structure of a β-(1→3)-D-Glucan from Yeast Cell Walls". Biochemical Journal. 135 (1): 19–30. doi:10.1042/bj1350019. PMC 1165784. PMID 4359920.
- Teas, J (1983). "The dietary intake of Laminarin, a brown seaweed, and breast cancer prevention". Nutrition and Cancer. 4 (3): 217–222. doi:10.1080/01635588209513760. ISSN 0163-5581. PMID 6302638.
- Vannucci, L; Krizan, J; Sima, P; Stakheev, D; Caja, F; Rajsiglova, L; Horak, V; Saieh, M (2013). "Immunostimulatory properties and antitumor activities of glucans (Review)". International Journal of Oncology. 43 (2): 357–64. doi:10.3892/ijo.2013.1974. PMC 3775562. PMID 23739801.
- McRorie Jr, J. W; McKeown, N. M (2017). "Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber". Journal of the Academy of Nutrition and Dietetics. 117 (2): 251–264. doi:10.1016/j.jand.2016.09.021. PMID 27863994.
- Keenan, M. J.; Martin, R. J.; Raggio, A. M.; McCutcheon, K. L.; Brown, I. L.; Birkett, A.; Newman, S. S.; Skaf, J.; Hegsted, M.; Tulley, R. T.; Blair, E.; Zhou, J. (2012). "High-Amylose Resistant Starch Increases Hormones and Improves Structure and Function of the Gastrointestinal Tract: A Microarray Study". Journal of Nutrigenetics and Nutrigenomics. 5 (1): 26–44. doi:10.1159/000335319. PMC 4030412. PMID 22516953.
- Simpson, H. L.; Campbell, B. J. (2015). "Review article: dietary fibre–microbiota interactions". Alimentary Pharmacology & Therapeutics. 42 (2): 158–79. doi:10.1111/apt.13248. PMC 4949558. PMID 26011307.
- Othman, R. A; Moghadasian, M. H; Jones, P. J (2011). "Cholesterol-lowering effects of oat β-glucan". Nutrition Reviews. 69 (6): 299–309. doi:10.1111/j.1753-4887.2011.00401.x. PMID 21631511.
- Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR (1 September 1996). "Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting". The Journal of Experimental Medicine. 184 (3): 1045–1059. doi:10.1084/jem.184.3.1045. PMC 2192803. PMID 9064322.
- Tsukagoshi S, Hashimoto Y, Fujii G, Kobayashi H, Nomoto K, Orita K (June 1984). "Krestin (PSK)". Cancer Treatment Reviews. 11 (2): 131–155. doi:10.1016/0305-7372(84)90005-7. PMID 6238674.
- Hong, F; Yan J; Baran JT; Allendorf DJ; Hansen RD; Ostroff GR; Xing PX; Cheung NK; Ross GD (15 July 2004). "Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models". Journal of Immunology. 173 (2): 797–806. doi:10.4049/jimmunol.173.2.797. ISSN 0022-1767. PMID 15240666.
- Obayashi T, Yoshida M, Mori T, et al. (1995). "Plasma (13)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes". Lancet. 345 (8941): 17–20. doi:10.1016/S0140-6736(95)91152-9. PMID 7799700. S2CID 27299444.
- Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. (2005). "Multicenter clinical evaluation of the (1→3)β-D-glucan assay as an aid to diagnosis of fungal infections in humans". Clin Infect Dis. 41 (5): 654–659. doi:10.1086/432470. PMID 16080087.
- Odabasi Z, Mattiuzzi G, Estey E, et al. (2004). "Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome". Clin Infect Dis. 39 (2): 199–205. doi:10.1086/421944. PMID 15307029.
- Mennink-Kersten MA, Warris A, Verweij PE (2006). "1,3-β-D-Glucan in patients receiving intravenous amoxicillin–clavulanic acid". NEJM. 354 (26): 2834–2835. doi:10.1056/NEJMc053340. PMID 16807428.
- Mennink-Kersten MA, Ruegebrink D, Verweij PE (2008). "Pseudomonas aeruginosa as a cause of 1,3-β-D-glucan assay reactivity". Clin Infect Dis. 46 (12): 1930–1931. doi:10.1086/588563. PMID 18540808.
- Lahmer, Tobias; da Costa, Clarissa Prazeres; Held, Jürgen; Rasch, Sebastian; Ehmer, Ursula; Schmid, Roland M.; Huber, Wolfgang (4 April 2017). "Usefulness of 1,3 Beta-D-Glucan Detection in non-HIV Immunocompromised Mechanical Ventilated Critically Ill Patients with ARDS and Suspected Pneumocystis jirovecii Pneumonia". Mycopathologia. 182 (7–8): 701–708. doi:10.1007/s11046-017-0132-x. ISSN 1573-0832. PMID 28378239. S2CID 3870306.
- He, Song; Hang, Ju-Ping; Zhang, Ling; Wang, Fang; Zhang, De-Chun; Gong, Fang-Hong (August 2015). "A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-D-glucan for invasive fungal infection: Focus on cutoff levels". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 48 (4): 351–361. doi:10.1016/j.jmii.2014.06.009. ISSN 1995-9133. PMID 25081986.
- Kullberg, Bart Jan; Arendrup, Maiken C. (8 October 2015). "Invasive Candidiasis". The New England Journal of Medicine. 373 (15): 1445–1456. doi:10.1056/NEJMra1315399. hdl:2066/152392. ISSN 1533-4406. PMID 26444731.
- Ostrosky-Zeichner, Luis; Alexander, Barbara D.; Kett, Daniel H.; Vazquez, Jose; Pappas, Peter G.; Saeki, Fumihiro; Ketchum, Paul A.; Wingard, John; Schiff, Robert (1 September 2005). "Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans". Clinical Infectious Diseases. 41 (5): 654–659. doi:10.1086/432470. ISSN 1537-6591. PMID 16080087.
External links
- beta-Glucans at the US National Library of Medicine Medical Subject Headings (MeSH)
