Chitin
Chitin (C8H13O5N)n (/ˈkaɪtɪn/ KY-tin) is a long-chain polymer of N-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chitin are produced each year in the biosphere.[1] It is a primary component of cell walls in fungi, the exoskeletons of arthropods such as crustaceans and insects, and the radulae, cephalopod beaks and gladii of molluscs. It is also synthesised by at least some fish and lissamphibians.[2] The structure of chitin is comparable to cellulose, forming crystalline nanofibrils or whiskers. It is functionally comparable to the protein keratin. Chitin has proved useful for several medicinal, industrial and biotechnological purposes.


Etymology
The English word "chitin" comes from the French word chitine, which was derived in 1821 from the Greek word χιτών (khitōn) meaning covering.[3]
A similar word, "chiton", refers to a marine animal with a protective shell.
Chemistry, physical properties and biological function

The structure of chitin was determined by Albert Hofmann in 1929. Hofmann hydrolyzed chitin using a crude preparation of the enzyme chitinase, which he obtained from the snail Helix pomatia.[4][5][6]
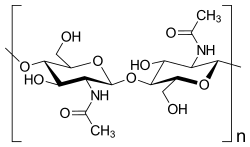
Chitin is a modified polysaccharide that contains nitrogen; it is synthesized from units of N-acetyl-D-glucosamine (to be precise, 2-(acetylamino)-2-deoxy-D-glucose). These units form covalent β-(1→4)-linkages (like the linkages between glucose units forming cellulose). Therefore, chitin may be described as cellulose with one hydroxyl group on each monomer replaced with an acetyl amine group. This allows for increased hydrogen bonding between adjacent polymers, giving the chitin-polymer matrix increased strength.

In its pure, unmodified form, chitin is translucent, pliable, resilient, and quite tough. In most arthropods, however, it is often modified, occurring largely as a component of composite materials, such as in sclerotin, a tanned proteinaceous matrix, which forms much of the exoskeleton of insects. Combined with calcium carbonate, as in the shells of crustaceans and molluscs, chitin produces a much stronger composite. This composite material is much harder and stiffer than pure chitin, and is tougher and less brittle than pure calcium carbonate.[7] Another difference between pure and composite forms can be seen by comparing the flexible body wall of a caterpillar (mainly chitin) to the stiff, light elytron of a beetle (containing a large proportion of sclerotin).[8]
In butterfly wing scales, chitin is organized into stacks of gyroids constructed of chitin photonic crystals that produce various iridescent colors serving phenotypic signaling and communication for mating and foraging.[9] The elaborate chitin gyroid construction in butterfly wings creates a model of optical devices having potential for innovations in biomimicry.[9] Scarab beetles in the genus Cyphochilus also utilize chitin to form extremely thin scales (five to fifteen micrometres thick) that diffusely reflect white light. These scales are networks of randomly ordered filaments of chitin with diameters on the scale of hundreds of nanometres, which serve to scatter light. The multiple scattering of light is thought to play a role in the unusual whiteness of the scales.[10][11] In addition, some social wasps, such as Protopolybia chartergoides, orally secrete material containing predominantly chitin to reinforce the outer nest envelopes, composed of paper.[12]
Chitosan is produced commercially by deacetylation of chitin; chitosan is soluble in water, while chitin is not.[13]
Nanofibrils have been made using chitin and chitosan.[14]
Humans and other mammals
Humans and other mammals have chitinase and chitinase-like proteins that can degrade chitin; they also possess several immune receptors that can recognize chitin and its degradation products, initiating an immune response.[15]
Chitin is sensed mostly in the lungs or gastrointestinal tract where it can activate the innate immune system through eosinophils or macrophages, as well as an adaptive immune response through T helper cells.[15] Keratinocytes in skin can also react to chitin or chitin fragments.[15]
Plants
Plants also have receptors that can cause a response to chitin, namely chitin elicitor receptor kinase 1 and chitin elicitor-binding protein.[15] The first chitin receptor was cloned in 2006.[16] When the receptors are activated by chitin, genes related to plant defense are expressed, and jasmonate hormones are activated, which in turn activate systematic defenses.[17] Commensal fungi have ways to interact with the host immune response that, as of 2016, were not well understood.[16]
Some pathogens produce chitin-binding proteins that mask the chitin they shed from these receptors.[17][18] Zymoseptoria tritici is an example of a fungal pathogen that has such blocking proteins; it is a major pest in wheat crops.[19]
Fossil record
Chitin was probably present in the exoskeletons of Cambrian arthropods such as trilobites. The oldest preserved chitin dates to the Oligocene, about 25 million years ago, consisting of a scorpion encased in amber.[20]
Uses
Agriculture
Chitin is a good inducer of plant defense mechanisms for controlling diseases.[21] It has potential for use as a soil fertilizer or conditioner to improve fertility and plant resilience that may enhance crop yields.[22][23]
Industrial
Chitin is used in many industrial processes. Examples of the potential uses of chemically modified chitin in food processing include the formation of edible films and as an additive to thicken and stabilize foods and food emulsions.[24][25] Processes to size and strengthen paper employ chitin and chitosan.[26][27]
Research
How chitin interacts with the immune system of plants and animals has been an active area of research, including the identity of key receptors with which chitin interacts, whether the size of chitin particles is relevant to the kind of immune response triggered, and mechanisms by which immune systems respond.[28][19] Chitin and chitosan have been explored as a vaccine adjuvant due to its ability to stimulate an immune response.[15]
Chitin and chitosan are under development as scaffolds in studies of how tissue grows and how wounds heal, and in efforts to invent better bandages, surgical thread, and materials for allotransplantation.[13][29] Sutures made of chitin have been explored for many years, but as of 2015, none were on the market; their lack of elasticity and problems making thread have prevented commercial development.[30]
In 2014, a method for using chitosan as a reproducible form of biodegradable plastic was introduced.[31] Chitin nanofibers are extracted from crustacean waste and mushrooms for possible development of products in tissue engineering, medicine, and industry.[32]
In 2020, chitin was proposed for use in building structures, tools, and other solid objects from a composite material of chitin combined with Martian regolith.[33] In this scenario, the biopolymers in the chitin act as the binder for the regolith aggregate to form a concrete-like composite material. The authors believe that waste materials from food production (e.g. scales from fish, exoskeletons from crustaceans and insects, etc.) could be put to use as feedstock for manufacturing processes.
See also
- Biopesticide
- Chitobiose
- Lorica
- Sporopollenin
- Tectin
References
- Nelson, D.L., Cox, M.M. (2017). Lehninger Principles of Biochemistry (7th ed.). McMillan Learning. ISBN 978-1-4641-2611-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Tang, WJ; Fernandez, JG; Sohn, JJ; Amemiya, CT (2015). "Chitin is endogenously produced in vertebrates". Curr Biol. 25 (7): 897–900. doi:10.1016/j.cub.2015.01.058. PMC 4382437. PMID 25772447.
- Odier, Auguste (1823). "Mémoire sur la composition chimique des parties cornées des insectes" [Memoir on the chemical composition of the horny parts of insects]. Mémoires de la Société d'Histoire Naturelle de Paris (in French). presented: 1821. 1: 29–42.
la Chitine (c'est ainsi que je nomme cette substance de chiton, χιτον, enveloppe… [chitine (it is thus that I name this substance from chiton, χιτον, covering)]"
- Hofmann, A. (1929). Über den enzymatischen Abbau des Chitins und Chitosans [On the enzymatic degradation of chitin and chitosan] (Thesis). Zurich, Switzerland: University of Zurich.
- Karrer, P.; Hofmann, A. (1929). "Polysaccharide XXXIX. Über den enzymatischen Abbau von Chitin and Chitosan I". Helvetica Chimica Acta (in German). 12 (1): 616–637. doi:10.1002/hlca.19290120167.
- Finney, Nathaniel S.; Siegel, Jay S. (2008). "In Memoriam: Albert Hofmann (1906-2008)" (PDF). Chimia. University of Zurich. 62 (5): 444–447. doi:10.2533/chimia.2008.444.
- Campbell, N. A. (1996) Biology (4th edition) Benjamin Cummings, New Work. p.69 ISBN 0-8053-1957-3
- Gilbert, Lawrence I. (2009). Insect development : morphogenesis, molting and metamorphosis. Amsterdam Boston: Elsevier/Academic Press. ISBN 978-0-12-375136-2.
- Saranathan V, Osuji CO, Mochrie SG, Noh H, Narayanan S, Sandy A, Dufresne ER, Prum RO (2010). "Structure, function, and self-assembly of single network gyroid (I4132) photonic crystals in butterfly wing scales". Proc Natl Acad Sci U S A. 107 (26): 11676–81. Bibcode:2010PNAS..10711676S. doi:10.1073/pnas.0909616107. PMC 2900708. PMID 20547870.
- Dasi Espuig M (16 August 2014). "Beetles' whiteness understood". BBC News: Science and Environment. Retrieved 15 November 2014.
- Burresi, Matteo; Cortese, Lorenzo; Pattelli, Lorenzo; Kolle, Mathias; Vukusic, Peter; Wiersma, Diederik S.; Steiner, Ullrich; Vignolini, Silvia (2014). "Bright-white beetle scales optimise multiple scattering of light". Scientific Reports. 4: 6075. Bibcode:2014NatSR...4E6075B. doi:10.1038/srep06075. PMC 4133710. PMID 25123449.
- Kudô, K.; Yamane, Sô.; Mateus, S.; Tsuchida, K.; Itô, Y.; Miyano, S.; Yamamoto, H.; Zucchi, R. (2001-10-01). "Nest materials and some chemical characteristics of nests of a New World swarm-founding polistine wasp, Polybia paulista (Hymenoptera Vespidae)". Ethology Ecology & Evolution. 13 (4): 351–360. doi:10.1080/08927014.2001.9522766. ISSN 0394-9370. S2CID 86452110.
- Bedian, L; Villalba-Rodríguez, AM; Hernández-Vargas, G; Parra-Saldivar, R; Iqbal, HM (May 2017). "Bio-based materials with novel characteristics for tissue engineering applications - A review". International Journal of Biological Macromolecules. 98: 837–846. doi:10.1016/j.ijbiomac.2017.02.048. PMID 28223133.
- Jeffryes, C; Agathos, SN; Rorrer, G (June 2015). "Biogenic nanomaterials from photosynthetic microorganisms". Current Opinion in Biotechnology. 33: 23–31. doi:10.1016/j.copbio.2014.10.005. PMID 25445544.
- Elieh Ali Komi, D; Sharma, L; Dela Cruz, CS (1 March 2017). "Chitin and Its Effects on Inflammatory and Immune Responses". Clinical Reviews in Allergy & Immunology. 54 (2): 213–223. doi:10.1007/s12016-017-8600-0. PMC 5680136. PMID 28251581.
- Sánchez-Vallet, A; Mesters, JR; Thomma, BP (March 2015). "The battle for chitin recognition in plant-microbe interactions". FEMS Microbiology Reviews. 39 (2): 171–83. doi:10.1093/femsre/fuu003. ISSN 0168-6445. PMID 25725011.
- Sharp, Russell G. (21 November 2013). "A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields". Agronomy. 3 (4): 757–793. doi:10.3390/agronomy3040757.
- Rovenich, H; Zuccaro, A; Thomma, BP (December 2016). "Convergent evolution of filamentous microbes towards evasion of glycan-triggered immunity". The New Phytologist. 212 (4): 896–901. doi:10.1111/nph.14064. PMID 27329426.
- Kettles, GJ; Kanyuka, K (15 April 2016). "Dissecting the Molecular Interactions between Wheat and the Fungal Pathogen Zymoseptoria tritici". Frontiers in Plant Science. 7: 508. doi:10.3389/fpls.2016.00508. PMC 4832604. PMID 27148331.
- Briggs, DEG (29 January 1999). "Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis". Philosophical Transactions of the Royal Society B: Biological Sciences. 354 (1379): 7–17. doi:10.1098/rstb.1999.0356. PMC 1692454.
- El Hadrami, A; Adam, L. R.; El Hadrami, I; Daayf, F (2010). "Chitosan in plant protection". Marine Drugs. 8 (4): 968–987. doi:10.3390/md8040968. PMC 2866471. PMID 20479963.
- Debode, Jane; De Tender, Caroline; Soltaninejad, Saman; Van Malderghem, Cinzia; Haegeman, Annelies; Van der Linden, Inge; Cottyn, Bart; Heyndrickx, Marc; Maes, Martine (2016-04-21). "Chitin mixed in potting soil alters lettuce growth, the survival of zoonotic bacteria on the leaves and associated rhizosphere microbiology". Frontiers in Microbiology. 7: 565. doi:10.3389/fmicb.2016.00565. ISSN 1664-302X. PMC 4838818. PMID 27148242.
- Sarathchandra, S. U.; Watson, R. N.; Cox, N. R.; di Menna, M. E.; Brown, J. A.; Burch, G.; Neville, F. J. (1996-05-01). "Effects of chitin amendment of soil on microorganisms, nematodes, and growth of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.)". Biology and Fertility of Soils. 22 (3): 221–226. doi:10.1007/BF00382516. ISSN 1432-0789. S2CID 32594901.
- Tzoumaki, Maria V.; Moschakis, Thomas; Kiosseoglou, Vassilios; Biliaderis, Costas G. (August 2011). "Oil-in-water emulsions stabilized by chitin nanocrystal particles". Food Hydrocolloids. 25 (6): 1521–1529. doi:10.1016/j.foodhyd.2011.02.008. ISSN 0268-005X.
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. (1999). "Food applications of chitin and chitosans". Trends in Food Science & Technology. 10 (2): 37–51. doi:10.1016/s0924-2244(99)00017-5.
- Hosokawa, Jun; Nishiyama, Masashi; Yoshihara, Kazutoshi; Kubo, Takamasa (May 1990). "Biodegradable film derived from chitosan and homogenized cellulose". Industrial & Engineering Chemistry Research. 29 (5): 800–805. doi:10.1021/ie00101a015. ISSN 0888-5885.
- Gällstedt, Mikael; Brottman, Angela; Hedenqvist, Mikael S. (July 2005). "Packaging-related properties of protein- and chitosan-coated paper". Packaging Technology and Science. 18 (4): 161–170. doi:10.1002/pts.685. ISSN 0894-3214. S2CID 96578009.
- Gómez-Casado, Cristina; Díaz-Perales, Araceli; Hedenqvist, Mikael S. (2016-10-01). "Allergen-Associated Immunomodulators: Modifying Allergy Outcome". Archivum Immunologiae et Therapiae Experimentalis. 64 (5): 339–347. doi:10.1007/s00005-016-0401-2. ISSN 1661-4917. PMID 27178664. S2CID 15221318.
- Cheung, R. C.; Ng, T. B.; Wong, J. H.; Chan, W. Y. (2015). "Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications". Marine Drugs. 13 (8): 5156–5186. doi:10.3390/md13085156. PMC 4557018. PMID 26287217.
- Ducheyne, Paul; Healy, Kevin; Hutmacher, Dietmar E.; Grainger, David W.; Kirkpatrick, C. James, eds. (2011). Comprehensive biomaterials. Amsterdam: Elsevier. p. 230. ISBN 9780080552941.
- "Harvard researchers develop bioplastic made from shrimp shells". Fox News. 16 May 2014. Retrieved 24 May 2014.
- Ifuku, Shinsuke (2014). "Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications". Molecules. 19 (11): 18367–80. doi:10.3390/molecules191118367. PMC 6271128. PMID 25393598.
- Shiwei, Ng; Dritsas, Stylianos; Fernandez, Javier G. (September 16, 2020). "Martian biolith: A bioinspired regolith composite for closed-loop extraterrestrial manufacturing". PLOS ONE. 15 (9): e0238606. Bibcode:2020PLoSO..1538606S. doi:10.1371/journal.pone.0238606. PMC 7494075. PMID 32936806.