Homogeneity and heterogeneity
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities.[1][2]
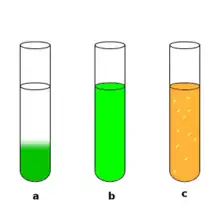
only 'b' is homogeneous
Etymology and spelling
The words homogeneous and heterogeneous come from Medieval Latin homogeneus and heterogeneus, from Ancient Greek ὁμογενής (homogenēs) and ἑτερογενής (heterogenēs), from ὁμός (homos, “same”) and ἕτερος (heteros, “other, another, different”) respectively, followed by γένος (genos, “kind”); -ous is an adjectival suffix.[3]
Alternate spellings omitting the last -e- (and the associated pronunciations) are common, but mistaken:[4] homogenous is strictly a biological/pathological term which has largely been replaced by homologous. But use of homogenous to mean homogeneous has seen a rise since 2000 sufficient enough for it to now be considered an "established variant".[5] Similarly, heterogenous is a spelling traditionally reserved to biology and pathology, referring to the property of an object in the body having its origin outside the body.[6]
Scaling
The concepts are the same to every level of complexity. From atoms to galaxies, plants, animals, humans, and other living organisms all share both a common or unique set of complexities. Hence, an element may be homogeneous on a larger scale, compared to being heterogeneous on a smaller scale. This is known as an effective medium approximation. [7][8]
Examples
Various disciplines understand heterogeneity, or being heterogeneous, in different ways.[2]
Chemistry
In chemistry, a heterogeneous mixture consists of either or both of 1) multiple states of matter or 2) hydrophilic and hydrophobic substances in one mixture; an example of the latter would be a mixture of water, octane, and silicone grease. Heterogeneous solids, liquids, and gases may be made homogeneous by melting, stirring, or by allowing time to pass for diffusion to distribute the molecules evenly. For example, adding dye to water will create a heterogeneous solution at first, but will become homogeneous over time. Entropy allows for heterogeneous substances to become homogeneous over time.
A heterogeneous mixture is a mixture of two or more compounds. Examples are: mixtures of sand and water or sand and iron filings, a conglomerate rock, water and oil, a salad, trail mix, and concrete (not cement).[9] A mixture can be determined to be homogeneous when everything is settled and equal, and the liquid, gas, the object is one color or the same form. Various models have been proposed to model the concentrations in different phases. The phenomena to be considered are mass rates and reaction.
Homogeneous and heterogeneous reactions
Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. Reactions that take place on the surface of a catalyst of a different phase are also heterogeneous. A reaction between two gases or two miscible liquids is homogeneous. A reaction between a gas and a liquid, a gas and a solid or a liquid and a solid is heterogeneous.
Geology
Earth is a heterogeneous substance in many aspects; for instance, rocks (geology) are inherently heterogeneous, usually occurring at the micro-scale and mini-scale.[7]
Information technology
With information technology, heterogeneous computing occurs in a network comprising different types of computers, potentially with vastly differing memory sizes, processing power and even basic underlying architecture.
Mathematics and statistics
In algebra, homogeneous polynomials have the same number of factors of a given kind.
In the study of binary relations, a homogeneous relation R is on a single set (R ⊆ X × X) while a heterogeneous relation concerns possibly distinct sets (R ⊆ X × Y, X = Y or X ≠ Y).[10]
In statistical meta-analysis, study heterogeneity is when multiple studies on an effect are measuring somewhat different effects due to differences in subject population, intervention, choice of analysis, experimental design, etc.; this can cause problems in attempts to summarize the meaning of the studies.
Medicine
In medicine and genetics, a genetic or allelic heterogeneous condition is one where the same disease or condition can be caused, or contributed to, by several factors, or in genetic terms, by varying or different genes or alleles.
In cancer research, cancer cell heterogeneity is thought to be one of the underlying reasons that make treatment of cancer difficult.[11]
Physics
In physics, "heterogeneous" is understood to mean "having physical properties that vary within the medium".
See also
- Complete spatial randomness
- Heterologous
- Epidemiology
- Spatial analysis
- Statistical hypothesis testing
- Homogeneity blockmodeling
References
- Heterogeneous Mixtures, in chemistry, is where certain elements are unwillingly combined and, when given the option, will separate. "Webster's Revised Unabridged Dictionary (1913 + 1828)". Heterogeneity. The ARTFL Project, University of Chicago. September 2010. Archived from the original (Part of this paragraph is public domain material copyright 1828 and 1913) on 2011-07-28. Retrieved 2010-09-10.
- "Webster's Revised Unabridged Dictionary (1913 + 1828)". Heterogeneous. The ARTFL Project, University of Chicago. September 2010. Archived from the original (Part of this paragraph is public domain material copyright 1828 and 1913) on 2011-07-28. Retrieved 2010-09-10.
- Heterogeneous: Definition in the Oxford English Dictionary
- Cambridge Dictionary: homogeneous
- Homogeneous: Definition in the Oxford England Dictionary
- Heterogeneous vs. heterogenous - Grammarist)
- Guéguen, Yves; Palciauskas, Victor (May 1994). Introduction to the physics of rocks. Princeton University Press. pp. 53–72 (Chapter 3). ISBN 978-0-691-03452-2.Google Books preview download available
- Shadrivov, Ilya V.; Kozyrev, AB; Van Der Weide, DW; Kivshar, YS (2008-11-24). "Nonlinear magnetic metamaterials" (Introduction section. Free PDF download). Optics Express. 16 (25): 20266–71. Bibcode:2008OExpr..1620266S. doi:10.1364/OE.16.020266. hdl:10440/410. PMID 19065165. Retrieved 2009-11-26.
- Gamow, George (April 1967). "Chapter VI, "Descending Staircase"". One Two Three... Infinity (Mass market paperback) (Bantam Science and Mathematics, 5th printing ed.). Bantam. p. 117.
[Clam chowder] represents a nice example of what is known as a heterogeneous material.
- Schmidt, Gunther; Ströhlein, Thomas (2012). [{{google%20books |plainurl=y |id=ZgarCAAAQBAJ|paged=277}} Relations and Graphs: Discrete Mathematics for Computer Scientists]. Definition 4.1.1.: Springer Science & Business Media. ISBN 978-3-642-77968-8.
{{cite book}}: Check|url=value (help)CS1 maint: location (link) - Bhatia, Sangeeta; John V Frangioni; Robert M Hoffman; A John Iafrate; Kornelia Polyak (10 July 2012). "The challenges posed by cancer heterogeneity". Nature Biotechnology. 30 (7): 604–610. doi:10.1038/nbt.2294. PMID 22781679. S2CID 15083285.
- Dictionary of Sociology. Routledge; 12 November 2012. ISBN 978-1-136-59845-6.
External links
- The following cited pages in this book cover the meaning of "homogeneity" across disciplines Morris, Christopher G. (1992). Academic Press Dictionary of Science and Technology. Gulf Professional Publishing. pp. 1039, 1040. ISBN 0-12-200400-0.
Homogeneity in physics.
.