Proprioception
Proprioception (/ˌproʊprioʊˈsɛpʃən, -priə-/[1][2] PROH-pree-o-SEP-shən), also referred to as kinaesthesia (or kinesthesia), is the sense of self-movement, force, and body position.[3][4] It is sometimes described as the "sixth sense".[5]
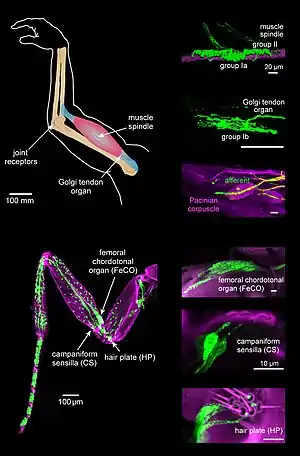
Proprioception is mediated by proprioceptors, mechanosensory neurons located within muscles, tendons, and joints.[3] Most animals possess multiple subtypes of proprioceptors, which detect distinct kinematic parameters, such as joint position, movement, and load. Although all mobile animals possess proprioceptors, the structure of the sensory organs can vary across species.
Proprioceptive signals are transmitted to the central nervous system, where they are integrated with information from other sensory systems, such as the visual system and the vestibular system, to create an overall representation of body position, movement, and acceleration. In many animals, sensory feedback from proprioceptors is essential for stabilizing body posture and coordinating body movement.
System overview
In vertebrates, limb velocity and movement (muscle length and the rate of change) are encoded by one group of sensory neurons (Type Ia sensory fiber) and another type encode static muscle length (Group II neurons).[6] These two types of sensory neurons compose muscle spindles. There is a similar division of encoding in invertebrates; different subgroups of neurons of the Chordotonal organ[7] encode limb position and velocity.
To determine the load on a limb, vertebrates use sensory neurons in the Golgi tendon organs:[8] type Ib afferents. These proprioceptors are activated at given muscle forces, which indicate the resistance that muscle is experiencing. Similarly, invertebrates have a mechanism to determine limb load: the Campaniform sensilla.[9] These proprioceptors are active when a limb experiences resistance.
A third role for proprioceptors is to determine when a joint is at a specific position. In vertebrates, this is accomplished by Ruffini endings and Pacinian corpuscles. These proprioceptors are activated when the joint is at a threshold, usually at the extremes of joint position. Invertebrates use hair plates[10] to accomplish this; a row of bristles located along joints detect when the limb moves.
Reflexes
The sense of proprioception is ubiquitous across mobile animals and is essential for the motor coordination of the body. Proprioceptors can form reflex circuits with motor neurons to provide rapid feedback about body and limb position. These mechanosensory circuits are important for flexibly maintaining posture and balance, especially during locomotion. For example, consider the stretch reflex, in which stretch across a muscle is detected by a sensory receptor (e.g., muscle spindle, chordotonal neurons), which activates a motor neuron to induce muscle contraction and oppose the stretch. During locomotion, sensory neurons can reverse their activity when stretched, to promote rather than oppose movement.[11][12]
Conscious and non-conscious
In humans, a distinction is made between conscious proprioception and non-conscious proprioception:
- Conscious proprioception is communicated by the dorsal column-medial lemniscus pathway to the cerebrum.[13]
- Non-conscious proprioception is communicated primarily via the dorsal spinocerebellar tract[14] and ventral spinocerebellar tract,[15] to the cerebellum.
- A non-conscious reaction is seen in the human proprioceptive reflex, or righting reflex—in the event that the body tilts in any direction, the person will cock their head back to level the eyes against the horizon.[16] This is seen even in infants as soon as they gain control of their neck muscles. This control comes from the cerebellum, the part of the brain affecting balance.
Mechanisms
Proprioception is mediated by mechanically sensitive proprioceptor neurons distributed throughout an animal's body. Most vertebrates possess three basic types of proprioceptors: muscle spindles, which are embedded in skeletal muscles, Golgi tendon organs, which lie at the interface of muscles and tendons, and joint receptors, which are low-threshold mechanoreceptors embedded in joint capsules. Many invertebrates, such as insects, also possess three basic proprioceptor types with analogous functional properties: chordotonal neurons, campaniform sensilla, and hair plates.[3]
The initiation of proprioception is the activation of a proprioceptor in the periphery.[17] The proprioceptive sense is believed to be composed of information from sensory neurons located in the inner ear (motion and orientation) and in the stretch receptors located in the muscles and the joint-supporting ligaments (stance). There are specific nerve receptors for this form of perception termed "proprioceptors", just as there are specific receptors for pressure, light, temperature, sound, and other sensory experiences. Proprioceptors are sometimes known as adequate stimuli receptors.
Members of the transient receptor potential family of ion channels have been found to be important for proprioception in fruit flies,[18] nematode worms,[19] African clawed frogs,[20] and zebrafish.[21] PIEZO2, a nonselective cation channel, has been shown to underlie the mechanosensitivity of proprioceptors in mice.[22] Humans with loss-of-function mutations in the PIEZO2 gene exhibit specific deficits in joint proprioception,[lower-alpha 1] as well as vibration and touch discrimination, suggesting that the PIEZO2 channel is essential for mechanosensitivity in some proprioceptors and low-threshold mechanoreceptors.[24]
Although it was known that finger kinesthesia relies on skin sensation, recent research has found that kinesthesia-based haptic perception relies strongly on the forces experienced during touch.[25] This research allows the creation of "virtual", illusory haptic shapes with different perceived qualities.[26]
Anatomy
Proprioception of the head stems from the muscles innervated by the trigeminal nerve, where the GSA fibers pass without synapsing in the trigeminal ganglion (first-order sensory neuron), reaching the mesencephalic tract and the mesencephalic nucleus of trigeminal nerve.[27] Proprioception of limbs often occurs due to receptors in connective tissue near joints.[28]
Function
Stability
An important role for proprioception is to allow an animal to stabilize itself against perturbations.[29] For instance, for a person to walk or stand upright, they must continuously monitor their posture and adjust muscle activity as needed to provide balance. Similarly, when walking on unfamiliar terrain or even tripping, the person must adjust the output of their muscles quickly based on estimated limb position and velocity. Proprioceptor reflex circuits are thought to play an important role to allow fast and unconscious execution of these behaviors, To make control of these behaviors efficient, proprioceptors are also thought to regulate reciprocal inhibition in muscles, leading to agonist-antagonist muscle pairs.
Planning and refining movements
When planning complex movements such as reaching or grooming, animals must consider the current position and velocity of their limb and use it to adjust dynamics to target a final position. If the animal's estimate of their limb's initial position is wrong, this can lead to a deficiency in the movement. Furthermore, proprioception is crucial in refining the movement if it deviates from the trajectory.
Development
In adult fruit flies, each proprioceptor class arises from a specific cell lineage (i.e. each chordotonal neuron is from the chordotonal neuron lineage, although multiple lineages give rise to sensory bristles). After the last cell division, proprioceptors send out axons toward the central nervous system and are guided by hormonal gradients to reach stereotyped synapses. [30] The mechanisms underlying axon guidance are similar across invertebrates and vertebrates.
In mammals with longer gestation periods, muscle spindles are fully formed at birth. Muscle spindles continue to grow throughout post-natal development as muscles grow. [31]
Mathematical models
Proprioceptors transfer the mechanical state of the body into patterns of neural activity. This transfer can be modeled mathematically, for example to better understand the internal workings of a proprioceptor[32][33][34] or to provide more realistic feedback in neuromechanical simulations.[35][36]
Various proprioceptor models of complexity have been developed. They range from simple phenomenological models to complex structural models, in which the mathematical elements correspond to anatomical features of the proprioceptor. The focus has been on muscle spindles,[32][33][34][37] but Golgi tendon organs[38][39] and insects hair plates[40] have been modeled too.
Muscle spindles
Poppelle and Bowman [41] used linear system theory to model mammalian muscle spindles Ia and II afferents. They obtained a set of de-afferented muscle spindles, measured their response to a series of sinusoidal and step function stretches, and fit a transfer function to the spike rate. They found that the following Laplace transfer function describes the firing rate responses of the primary sensory fibers for a change in length:
The following equation describes the response of secondary sensory fibers:
More recently, Blum et al. [42] showed that the muscle spindle firing rate is modeled better as tracking the force of the muscle, rather than the length. Furthermore, muscle spindle firing rates show history dependence which cannot be modeled by a linear time-invariant system model.
Golgi tendon organs
Houk and Simon [39] provided one of the first mathematical models of a Golgi tendon organ receptor, modeling the firing rate of the receptor as a function of the muscle tension force. Just as for muscle spindles, they find that, as the receptors respond linearly to sine waves of different frequencies and has little variance in response over time to the same stimulus, Golgi tendon organ receptors may be modeled as linear time-invariant systems. Specifically, they find that the firing rate of a Golgi tendon organ receptor may be modeled as a sum of 3 decaying exponentials:
where is the firing rate and is a step function of force.
The corresponding Laplace transfer function for this system is:
For a soleus receptor, Houk and Simon obtain average values of K=57 pulses/sec/kg, A=0.31, a=0.22 sec−1, B=0.4, b=2.17 sec−1, C=2.5, c=36 sec−1 .
When modeling a stretch reflex, Lin and Crago[43] improved upon this model by adding a logarithmic nonlinearity before the Houk and Simon model and a threshold nonlinearity after.
Impairment
Permanent
Proprioception is permanently impaired in patients with joint hypermobility or Ehlers-Danlos syndrome (a genetic condition that results in weak connective tissue throughout the body).[44] It can also be permanently impaired from viral infections as reported by Sacks. The catastrophic effect of major proprioceptive loss is reviewed by Robles-De-La-Torre (2006).[45]
Proprioception is also permanently impaired in physiological aging (presbypropria)[46] and autism spectrum disorder.[47]
Parkinson's disease is characterized by a decline in motor function as a result of neurodegeneration. It is likely that some of the symptoms of Parkinson's disease are in part related to disrupted proprioception.[48] Whether this symptom is caused by degeneration of proprioceptors in the periphery or disrupted signaling in the brain or spinal cord is an open question.
People who have a limb amputated may still have a confused sense of that limb's existence on their body, known as phantom limb syndrome. Phantom sensations can occur as passive proprioceptive sensations of the limb's presence, or more active sensations such as perceived movement, pressure, pain, itching, or temperature. There are a variety of theories concerning the etiology of phantom limb sensations and experience. One is the concept of "proprioceptive memory", which argues that the brain retains a memory of specific limb positions and that after amputation there is a conflict between the visual system, which actually sees that the limb is missing, and the memory system which remembers the limb as a functioning part of the body.[49] Phantom sensations and phantom pain may also occur after the removal of body parts other than the limbs, such as after amputation of the breast, extraction of a tooth (phantom tooth pain), or removal of an eye (phantom eye syndrome).
Temporary
Proprioception is occasionally impaired spontaneously, especially when one is tired. Similar effects can be felt during the hypnagogic state of consciousness, during the onset of sleep. One's body may feel too large or too small, or parts of the body may feel distorted in size. Similar effects can sometimes occur during epilepsy or migraine auras. These effects are presumed to arise from abnormal stimulation of the part of the parietal cortex of the brain involved with integrating information from different parts of the body.[50] Proprioceptive illusions can also be induced, such as the Pinocchio illusion.
Temporary impairment of proprioception has also been known to occur from an overdose of vitamin B6 (pyridoxine and pyridoxamine). Most of the impaired function returns to normal shortly after the amount of the vitamin in the body returns to a level that is closer to that of the physiological norm. Impairment can also be caused by cytotoxic factors such as chemotherapy.
It has been proposed that even common tinnitus and the attendant hearing frequency-gaps masked by the perceived sounds may cause erroneous proprioceptive information to the balance and comprehension centers of the brain, precipitating mild confusion.
Temporary loss or impairment of proprioception may happen periodically during growth, mostly during adolescence. Growth that might also influence this would be large increases or drops in bodyweight/size due to fluctuations of fat (liposuction, rapid fat loss or gain) and/or muscle content (bodybuilding, anabolic steroids, catabolisis/starvation). It can also occur in those that gain new levels of flexibility, stretching, and contortion. A limb's being in a new range of motion never experienced (or at least, not for a long time since youth perhaps) can disrupt one's sense of location of that limb. Possible experiences include suddenly feeling that feet or legs are missing from one's mental self-image; needing to look down at one's limbs to be sure they are still there; and falling down while walking, especially when attention is focused upon something other than the act of walking.
Diagnosis
Impaired proprioception may be diagnosed through a series of tests, each focusing on a different functional aspect of proprioception.
The Romberg's test is often used to assess balance. The subject must stand with feet together and eyes closed without support for 30 seconds. If the subject loses balance and falls, it is an indicator for impaired proprioception.
For evaluating proprioception's contribution to motor control, a common protocol is joint position matching.[51] The patient is blindfolded while a joint is moved to a specific angle for a given period of time and then returned to neutral. The subject is then asked to move the joint back to the specified angle. Recent investigations have shown that hand dominance, participant age, active versus passive matching, and presentation time of the angle can all affect performance on joint position matching tasks.
For passive sensing of joint angles, recent studies have found that experiments to probe psychophysical thresholds produce more precise estimates of proprioceptive discrimination than the joint position matching task.[52] In these experiments, the subject holds on to an object (such as an armrest) that moves and stops at different positions. The subject must discriminate whether one position is closer to the body than another. From the subject's choices, the tester may determine the subject's discrimination thresholds.
Proprioception is tested by American police officers using the field sobriety testing to check for alcohol intoxication. The subject is required to touch his or her nose with eyes closed; people with normal proprioception may make an error of no more than 20 mm (0.79 in), while people with impaired proprioception (a symptom of moderate to severe alcohol intoxication) fail this test due to difficulty locating their limbs in space relative to their noses.
Training
Proprioception is what allows someone to learn to walk in complete darkness without losing balance. During the learning of any new skill, sport, or art, it is usually necessary to become familiar with some proprioceptive tasks specific to that activity. Without the appropriate integration of proprioceptive input, an artist would not be able to brush paint onto a canvas without looking at the hand as it moved the brush over the canvas; it would be impossible to drive an automobile because a motorist would not be able to steer or use the pedals while looking at the road ahead; a person could not touch type or perform ballet; and people would not even be able to walk without watching where they put their feet.
Oliver Sacks reported the case of a young woman who lost her proprioception due to a viral infection of her spinal cord.[53] At first she could not move properly at all or even control her tone of voice (as voice modulation is primarily proprioceptive). Later she relearned by using her sight (watching her feet) and inner ear only for movement while using hearing to judge voice modulation. She eventually acquired a stiff and slow movement and nearly normal speech, which is believed to be the best possible in the absence of this sense. She could not judge effort involved in picking up objects and would grip them painfully to be sure she did not drop them.
The proprioceptive sense can be sharpened through study of many disciplines. Juggling trains reaction time, spatial location, and efficient movement. Standing on a wobble board or balance board is often used to retrain or increase proprioceptive abilities, particularly as physical therapy for ankle or knee injuries. Slacklining is another method to increase proprioception.
Standing on one leg (stork standing) and various other body-position challenges are also used in such disciplines as yoga, Wing Chun and tai chi.[54] The vestibular system of the inner ear, vision and proprioception are the main three requirements for balance.[55] Moreover, there are specific devices designed for proprioception training, such as the exercise ball, which works on balancing the abdominal and back muscles.
History of study
In 1557, the position-movement sensation was described by Julius Caesar Scaliger as a "sense of locomotion".[56]
In 1826, Charles Bell expounded the idea of a "muscle sense",[57] which is credited as one of the first descriptions of physiologic feedback mechanisms.[58] Bell's idea was that commands are carried from the brain to the muscles, and that reports on the muscle's condition would be sent in the reverse direction.
In 1847, the London neurologist Robert Todd highlighted important differences in the anterolateral and posterior columns of the spinal cord, and suggested that the latter were involved in the coordination of movement and balance.[59]
At around the same time, Moritz Heinrich Romberg, a Berlin neurologist, was describing unsteadiness made worse by eye closure or darkness, now known as the eponymous Romberg's sign, once synonymous with tabes dorsalis, that became recognised as common to all proprioceptive disorders of the legs.
In 1880, Henry Charlton Bastian suggested "kinaesthesia" instead of "muscle sense" on the basis that some of the afferent information (back to the brain) comes from other structures, including tendons, joints, and skin.[60]
In 1889, Alfred Goldscheider suggested a classification of kinaesthesia into three types: muscle, tendon, and articular sensitivity.[61]
In 1906, the term proprio-ception (and also intero-ception and extero-ception) is attested in a publication by Charles Scott Sherrington involving receptors.[62] He explains the terminology as follows:[63]
The main fields of distribution of the receptor organs fundamentally distinguishable seem, therefore, to be two, namely, a surface field constituted by the surface layer of the organism, and a deep field constituted by the tissues of the organism beneath the surface sheet.
[...]
the stimulations occurring in [the] deep field is that the stimuli are traceable to actions of the organism itself, and are so in much greater measure than are the stimulations of the surface field of the organism. Since in the deep field the stimuli to the receptors are delivered by the organism itself,[lower-alpha 2] the deep receptors may be termed proprio-ceptors, and the deep field a field of proprio-ception.
Today, the "exteroceptors" are the organs that provide information originating outside the body, such as the eyes, ears, mouth, and skin. The interoceptors provide information about the internal organs, and the "proprioceptors" provide information about movement derived from muscular, tendon, and articular sources. Using Sherrington's system, physiologists and anatomists search for specialised nerve endings that transmit mechanical data on joint capsule, tendon and muscle tension (such as Golgi tendon organs and muscle spindles), which play a large role in proprioception.
Primary endings of muscle spindles "respond to the size of a muscle length change and its speed" and "contribute both to the sense of limb position and movement".[64] Secondary endings of muscle spindles detect changes in muscle length, and thus supply information regarding only the sense of position.[64] Essentially, muscle spindles are stretch receptors.[65] It has been accepted that cutaneous receptors also contribute directly to proprioception by providing "accurate perceptual information about joint position and movement", and this knowledge is combined with information from the muscle spindles.[66]
Etymology
Proprioception is from Latin proprius, meaning "one's own", "individual", and capio, capere, to take or grasp. Thus to grasp one's own position in space, including the position of the limbs in relation to each other and the body as a whole.
The word kinesthesia or kinæsthesia (kinesthetic sense) refers to movement sense, but has been used inconsistently to refer either to proprioception alone or to the brain's integration of proprioceptive and vestibular inputs. Kinesthesia is a modern medical term composed of elements from Greek; kinein "to set in motion; to move" (from PIE root *keie- "to set in motion") + aisthesis "perception, feeling" (from PIE root *au- "to perceive") + Greek abstract noun ending -ia (corresponds to English -hood e.g. motherhood).
Plants and bacteria
Although they lack neurons, a form of proprioception has also been described in some plants (angiosperms).[67][68] Terrestrial plants control the orientation of their primary growth through the sensing of several vectorial stimuli such as the light gradient or the gravitational acceleration. This control has been called tropism. A quantitative study of shoot gravitropism demonstrated that, when a plant is tilted, it cannot recover a steady erected posture under the sole driving of the sensing of its angular deflection versus gravity. An additional control through the continuous sensing of its curvature by the organ and the subsequent driving an active straightening process are required.[67][68][69] Being a sensing by the plant of the relative configuration of its parts, it has been called proprioception. This dual sensing and control by gravisensing and proprioception has been formalized into a unifying mathematical model simulating the complete driving of the gravitropic movement. This model has been validated on 11 species sampling the phylogeny of land angiosperms, and on organs of very contrasted sizes, ranging from the small germination of wheat (coleoptile) to the trunk of poplar trees.[67][68]
Further studies have shown that the cellular mechanism of proprioception in plants involves myosin and actin, and seems to occur in specialized cells.[70] Proprioception was then found to be involved in other tropisms and to be central also to the control of nutation.[71]
The discovery of proprioception in plants has generated an interest in the popular science and generalist media.[72][73] This is because this discovery questions a long-lasting a priori that we have on plants. In some cases this has led to a shift between proprioception and self-awareness or self-consciousness. There is no scientific ground for such a semantic shift. Indeed, even in animals, proprioception can be unconscious; so it is thought to be in plants.[68][73]
Preliminary research indicates that bacteria may display proprioception.[74]
See also
- Balance disorder – Physiological disturbance of perception
- Body image – Person's perception of the aesthetics or sexual attractiveness of their own body
- Body schema – Postural model that keeps track of limb position
- Broken escalator phenomenon – The sensation of losing balance or dizziness when stepping onto an escalator which is not working
- Dizziness – Neurological condition causing impairment in spatial perception and stability
- Equilibrioception
- Eye-hand coordination
- Ideomotor phenomenon – Concept in hypnosis and psychological research
- Illusions of self-motion – Misperception of one's location or movement
- Instinctive aiming
- Kinaesthetics
- Kinesthetic learning – Learning by physical activities
- Motion sickness – Nausea caused by motion or perceived motion
- Motor control – Regulation of movement within organisms possessing a nervous system
- Multisensory integration – Study of senses and nervous system
- Seasickness – Motion sickness occurring at sea
- Spatial disorientation – Inability of a person to correctly determine their body position in space
- Theory of multiple intelligences – Theory of intelligence proposed by Howard Gardner
- Vertigo – Type of dizziness where a person has the sensation of moving or surrounding objects moving
Notes
References
- "Proprioception". Merriam-Webster Dictionary.
- "proprioceptive – definition of proprioceptive in English from the Oxford dictionary". OxfordDictionaries.com. Archived from the original on September 3, 2012. Retrieved 2016-01-20.
- Tuthill JC, Azim E (1 March 2018). "Proprioception". Current Biology. 28 (5): R194–R203. doi:10.1016/j.cub.2018.01.064. PMID 29510103.
- Balasubramanian, Ravi; Santos, Veronica (3 January 2014). The Human Hand as an Inspiration for Robot Hand Development. Springer. p. 127. ISBN 978-3-319-03017-3.
Proprioception also includes the ability to perceive force and heaviness, the history of which has been less controversial than the senses of limb position and movement. The sense of force refers to the ability to perceive the force that is generated by the muscles and its primary receptor is the Golgi tendon organ.
- Gandevia S, Proske U (1 September 2016). "Proprioception: The Sense Within". The Scientist. Retrieved 25 July 2018.
- Lundberg A, Malmgren K, Schomburg ED (November 1978). "Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents". The Journal of Physiology. 284: 327–43. doi:10.1113/jphysiol.1978.sp012543. PMC 1282824. PMID 215758.
- Bush BM (April 1965). "Proprioception by the Coxo-Basal Chordotonal Organ, Cb, in Legs of the Crab, Carcinus Maenas". The Journal of Experimental Biology. 42 (2): 285–97. doi:10.1242/jeb.42.2.285. PMID 14323766.
- Murphy JT, Wong YC, Kwan HC (July 1975). "Afferent-efferent linkages in motor cortex for single forelimb muscles". Journal of Neurophysiology. 38 (4): 990–1014. doi:10.1152/jn.1975.38.4.990. PMID 125786. S2CID 20111229.
- Chapman KM (April 1965). "Campaniform Sensilla on the Tactile Spines of the Legs of the Cockroach". The Journal of Experimental Biology. 42 (2): 191–203. doi:10.1242/jeb.42.2.191. PMID 14323763.
- Bräunig P, Hustert R, Pflüger HJ (1981). "Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. I. Morphology, location and innervation of internal proprioceptors of pro- and metathorax and their central projections". Cell and Tissue Research. 216 (1): 57–77. doi:10.1007/bf00234545. PMID 7226209. S2CID 29439820.
- Bässler U, Büschges A (June 1998). "Pattern generation for stick insect walking movements--multisensory control of a locomotor program". Brain Research. Brain Research Reviews. 27 (1): 65–88. doi:10.1016/S0165-0173(98)00006-X. PMID 9639677. S2CID 16673654.
- Tuthill JC, Wilson RI (October 2016). "Mechanosensation and Adaptive Motor Control in Insects". Current Biology. 26 (20): R1022–R1038. doi:10.1016/j.cub.2016.06.070. PMC 5120761. PMID 27780045.
- Fix JD (2002). Neuroanatomy. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 127. ISBN 978-0-7817-2829-4.
- Swenson RS. "Review of Clinical and Functional Neuroscience, Chapter 7A: Somatosensory Systems". (online version Dartmouth college). Archived from the original on 2008-04-05. Retrieved 2008-04-10.
- Siegel A (2010). Essential Neuroscience. Lippincott Williams & Wilkins. p. 263.
- "TMJ, Forward Head Posture and Neck Pain". Freedom From Pain Institute. Archived from the original on 2013-10-05. Retrieved 3 October 2013.
- Sherrington CS (1907). "On the proprioceptive system, especially in its reflex aspect". Brain. 29 (4): 467–85. doi:10.1093/brain/29.4.467.
- Walker RG, Willingham AT, Zuker CS (March 2000). "A Drosophila mechanosensory transduction channel". Science. 287 (5461): 2229–34. Bibcode:2000Sci...287.2229W. CiteSeerX 10.1.1.646.2497. doi:10.1126/science.287.5461.2229. PMID 10744543.
- Li W, Feng Z, Sternberg PW, Xu XZ (March 2006). "A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue". Nature. 440 (7084): 684–87. Bibcode:2006Natur.440..684L. doi:10.1038/nature04538. PMC 2865900. PMID 16572173.
- Shin JB, Adams D, Paukert M, Siba M, Sidi S, Levin M, et al. (August 2005). "Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells". Proceedings of the National Academy of Sciences of the United States of America. 102 (35): 12572–77. Bibcode:2005PNAS..10212572S. doi:10.1073/pnas.0502403102. PMC 1194908. PMID 16116094.
- Sidi S, Friedrich RW, Nicolson T (July 2003). "NompC TRP channel required for vertebrate sensory hair cell mechanotransduction". Science. 301 (5629): 96–99. Bibcode:2003Sci...301...96S. doi:10.1126/science.1084370. PMID 12805553. S2CID 23882972.
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, et al. (December 2015). "Piezo2 is the principal mechanotransduction channel for proprioception". Nature Neuroscience. 18 (12): 1756–62. doi:10.1038/nn.4162. PMC 4661126. PMID 26551544.
- The Nobel Assembly at Karolinska Institutet (4 Oct 2021) Press release: The Nobel Prize in Physiology or Medicine 2021 The Nobel Prize in Physiology or Medicine 2021: David Julius, and Ardem Patapoutian
- Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. (October 2016). "The Role of PIEZO2 in Human Mechanosensation". The New England Journal of Medicine. 375 (14): 1355–64. doi:10.1056/NEJMoa1602812. PMC 5911918. PMID 27653382.
- Robles-De-La-Torre G, Hayward V (July 2001). "Force can overcome object geometry in the perception of shape through active touch" (PDF). Nature. 412 (6845): 445–48. Bibcode:2001Natur.412..445R. doi:10.1038/35086588. PMID 11473320. S2CID 4413295. Archived from the original (PDF) on 2006-10-03. Retrieved 2006-10-03.
- the MIT Technology Review article "The Cutting Edge of Haptics"
- Orhan E. Arslan (7 August 2014). Neuroanatomical Basis of Clinical Neurology (2 ed.). CRC Press. pp. 432–. ISBN 978-1-4398-4834-0.
- van der Wal, Jaap (7 December 2009). "The Architecture of the Connective Tissue in the Musculoskeletal System—An Often Overlooked Functional Parameter as to Proprioception in the Locomotor Apparatus". International Journal of Therapeutic Massage & Bodywork. 2 (4): 9–23. doi:10.3822/ijtmb.v2i4.62. ISSN 1916-257X. PMC 3091473. PMID 21589740.
- David J. Magee; James E. Zachazewski; William S. Quillen (18 September 2008). Pathology and Intervention in Musculoskeletal Rehabilitation - E-Book. Elsevier Health Sciences. pp. 533–. ISBN 978-1-4160-6942-3. OCLC 1017975705.
- Jan, Y. N. and Jan, L. Y. (1993). The peripheral nervous system. In: The Development of Drosophila melanogaster (ed. Bate, M and Arias, A. M.), pp. 1207–44. New York: Cold Spring Harbor Laboratory Press.
- Maier A (February 1997). "Development and regeneration of muscle spindles in mammals and birds". The International Journal of Developmental Biology. 41 (1): 1–17. PMID 9074933.
- Blum KP, Lamotte D'Incamps B, Zytnicki D, Ting LH (September 2017). "Force encoding in muscle spindles during stretch of passive muscle". PLOS Computational Biology. 13 (9): e1005767. Bibcode:2017PLSCB..13E5767B. doi:10.1371/journal.pcbi.1005767. PMC 5634630. PMID 28945740.
- Mileusnic MP, Brown IE, Lan N, Loeb GE (October 2006). "Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle". Journal of Neurophysiology. 96 (4): 1772–88. doi:10.1152/jn.00868.2005. PMID 16672301.
- Maltenfort MG, Burke RE (May 2003). "Spindle model responsive to mixed fusimotor inputs and testable predictions of beta feedback effects". Journal of Neurophysiology. 89 (5): 2797–809. doi:10.1152/jn.00942.2002. PMID 12740414. S2CID 18253128.
- Röhrle O, Yavuz UŞ, Klotz T, Negro F, Heidlauf T (November 2019). "Multiscale modeling of the neuromuscular system: Coupling neurophysiology and skeletal muscle mechanics". Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 11 (6): e1457. doi:10.1002/wsbm.1457. PMID 31237041. S2CID 195354352.
- Prilutsky BI, Klishko AN, Weber DJ, Lemay MA (2016). "Computing Motion Dependent Afferent Activity During Cat Locomotion Using a Forward Dynamics Musculoskeletal Model". In Prilutsky BI, Edwards DH (eds.). Neuromechanical Modeling of Posture and Locomotion. Springer Series in Computational Neuroscience. New York: Springer. pp. 273–307. doi:10.1007/978-1-4939-3267-2_10. ISBN 978-1-4939-3267-2.
- Prochazka A (1999). Binder MD (ed.). Chapter 11 Quantifying Proprioception. Progress in Brain Research. Peripheral and Spinal Mechanisms in the Neural Control of Movement. Vol. 123. Elsevier. pp. 133–42.
- Mileusnic MP, Loeb GE (October 2006). "Mathematical models of proprioceptors. II. Structure and function of the Golgi tendon organ". Journal of Neurophysiology. 96 (4): 1789–802. doi:10.1152/jn.00869.2005. PMID 16672300.
- Houk J, Simon W (November 1967). "Responses of Golgi tendon organs to forces applied to muscle tendon". Journal of Neurophysiology. 30 (6): 1466–81. doi:10.1152/jn.1967.30.6.1466. PMID 6066449.
- Ache JM, Dürr V (July 2015). "A Computational Model of a Descending Mechanosensory Pathway Involved in Active Tactile Sensing". PLOS Computational Biology. 11 (7): e1004263. Bibcode:2015PLSCB..11E4263A. doi:10.1371/journal.pcbi.1004263. PMC 4497639. PMID 26158851.
- Poppele RE, Bowman RJ (January 1970). "Quantitative description of linear behavior of mammalian muscle spindles". Journal of Neurophysiology. 33 (1): 59–72. doi:10.1152/jn.1970.33.1.59. PMID 4243791.
- Blum KP, Lamotte D'Incamps B, Zytnicki D, Ting LH (September 2017). Ayers J (ed.). "Force encoding in muscle spindles during stretch of passive muscle". PLOS Computational Biology. 13 (9): e1005767. Bibcode:2017PLSCB..13E5767B. doi:10.1371/journal.pcbi.1005767. PMC 5634630. PMID 28945740.
- Lin, Chou-Ching K.; Crago, Patrick E. (January 2002). "Neural and Mechanical Contributions to the Stretch Reflex: A Model Synthesis". Annals of Biomedical Engineering. 30 (1): 54–67. doi:10.1114/1.1432692. ISSN 0090-6964. PMID 11874142. S2CID 13015209.
- Castori M (2012). "Ehlers-danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations". ISRN Dermatology. 2012: 751768. doi:10.5402/2012/751768. PMC 3512326. PMID 23227356.
- Robles-De-La-Torre G (2006). "The Importance of the Sense of Touch in Virtual and Real Environments" (PDF). IEEE MultiMedia. 13 (3): 24–30. doi:10.1109/MMUL.2006.69. S2CID 16153497. Archived from the original (PDF) on 2014-01-24. Retrieved 2006-10-07.
- Boisgontier MP, Olivier I, Chenu O, Nougier V (October 2012). "Presbypropria: the effects of physiological ageing on proprioceptive control". Age. 34 (5): 1179–94. doi:10.1007/s11357-011-9300-y. PMC 3448996. PMID 21850402.
- Blanche, Erna Imperatore; Reinoso, Gustavo; Chang, Megan C; Bodison, Stephanie (1 September 2012). "Proprioceptive Processing Difficulties Among Children With Autism Spectrum Disorders and Developmental Disabilities". The American Journal of Occupational Therapy. 66 (5): 621–624. doi:10.5014/ajot.2012.004234. PMC 3754787. PMID 22917129.
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M (November 2009). "Proprioception and motor control in Parkinson's disease". Journal of Motor Behavior. 41 (6): 543–52. doi:10.3200/35-09-002. PMID 19592360. S2CID 5775266.
- Weeks SR, Anderson-Barnes VC, Tsao JW (September 2010). "Phantom limb pain: theories and therapies" (PDF). The Neurologist. 16 (5): 277–86. doi:10.1097/nrl.0b013e3181edf128. PMID 20827116. S2CID 205894711. Archived from the original (PDF) on 2011-08-12.
- Ehrsson HH, Kito T, Sadato N, Passingham RE, Naito E (December 2005). "Neural substrate of body size: illusory feeling of shrinking of the waist". PLOS Biology. 3 (12): e412. doi:10.1371/journal.pbio.0030412. PMC 1287503. PMID 16336049.
- Goble DJ, Noble BC, Brown SH (August 2010). "Where was my arm again? Memory-based matching of proprioceptive targets is enhanced by increased target presentation time" (PDF). Neuroscience Letters. 481 (1): 54–58. doi:10.1016/j.neulet.2010.06.053. PMID 20600603. S2CID 24385107. Archived from the original (PDF) on 2014-12-19. Retrieved 2013-03-15.
- Elangovan, Naveen; Herrmann, Amanda; Konczak, Jürgen (April 2014). "Assessing proprioceptive function: evaluating joint position matching methods against psychophysical thresholds". Physical Therapy. 94 (4): 553–561. doi:10.2522/ptj.20130103. ISSN 1538-6724. PMC 6281037. PMID 24262599.
- Sacks, O. "The Disembodied Lady", in The Man Who Mistook His Wife for a Hat and his autobiographical case study A Leg to Stand On.
- cheng man ch'ing (1981). T'ai Chi Ch'uan. Blue Snake Books usa. pp. 86, 88. ISBN 978-0-913028-85-8.
- Hanc J (15 September 2010). "Staying on Balance, With the Help of Exercises". The New York Times. Archived from the original on 2017-10-11. Retrieved 11 October 2017.
- Jerosch J, Heisel J (2010). Management der Arthrose: Innovative Therapiekonzepte (in German). Deutscher Ärzteverlag. p. 107. ISBN 978-3-7691-0599-5. Retrieved 8 April 2011.
- Singh AK (1991). The Comprehensive History of Psychology. Motilal Banarsidass. p. 66. ISBN 978-81-208-0804-1. Retrieved 8 April 2011.
- Dickinson J (1976). Proprioceptive control of human movement. Princeton Book Co. p. 4. Retrieved 8 April 2011.
- Todd RB (1847). The Cyclopaedia of Anatomy and Physiology Vol. 4. London: Longmans. pp. 585–723.
- Foster SL (2010). Choreographing Empathy: Kinesthesia in Performance. Taylor & Francis. p. 74. ISBN 978-0-415-59655-8. Retrieved 8 April 2011.
- Brookhart JM, Mountcastle VB, Geiger SR (1984). Darian-Smith I (ed.). The Nervous system: Sensory processes;. American Physiological Society. p. 784. ISBN 978-0-683-01108-1. Retrieved 8 April 2011.
- Sherrington, C.S. (1906). The Integrative Action of the Nervous System. NewHaven, CT: Yale University Press.
- Sherrington, Charles Scott (1907). "On The Proprio-ceptive System, Especially In Its Reflex Aspect". Brain. 29 (4): 467–482. doi:10.1093/brain/29.4.467.
- Proske U, Gandevia SC (September 2009). "The kinaesthetic senses". The Journal of Physiology. 587 (Pt 17): 4139–46. doi:10.1113/jphysiol.2009.175372. PMC 2754351. PMID 19581378.
- Winter JA, Allen TJ, Proske U (November 2005). "Muscle spindle signals combine with the sense of effort to indicate limb position". The Journal of Physiology. 568 (Pt 3): 1035–46. doi:10.1113/jphysiol.2005.092619. PMC 1464181. PMID 16109730.
- Collins DF, Refshauge KM, Todd G, Gandevia SC (September 2005). "Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee". Journal of Neurophysiology. 94 (3): 1699–706. doi:10.1152/jn.00191.2005. PMID 15917323.
- Bastien R, Bohr T, Moulia B, Douady S (January 2013). "Unifying model of shoot gravitropism reveals proprioception as a central feature of posture control in plants". Proceedings of the National Academy of Sciences of the United States of America. 110 (2): 755–60. Bibcode:2013PNAS..110..755B. doi:10.1073/pnas.1214301109. PMC 3545775. PMID 23236182.
- Hamant O, Moulia B (October 2016). "How do plants read their own shapes?". The New Phytologist. 212 (2): 333–37. doi:10.1111/nph.14143. PMID 27532273.
- "From gravitropism to dynamical posture control: proprioception in plants". University of Cambridge. Archived from the original on 2017-08-05. Retrieved 5 August 2017.
- Okamoto K, Ueda H, Shimada T, Tamura K, Kato T, Tasaka M, et al. (March 2015). "Regulation of organ straightening and plant posture by an actin-myosin XI cytoskeleton". Nature Plants. 1 (4): 15031. doi:10.1038/nplants.2015.31. hdl:2433/197219. PMID 27247032. S2CID 22432635.
- Bastien R, Meroz Y (December 2016). "The Kinematics of Plant Nutation Reveals a Simple Relation between Curvature and the Orientation of Differential Growth". PLOS Computational Biology. 12 (12): e1005238. arXiv:1603.00459. Bibcode:2016PLSCB..12E5238B. doi:10.1371/journal.pcbi.1005238. PMC 5140061. PMID 27923062.
- Gabbatiss J (10 January 2017). "Plants can see, hear and smell – and respond". Archived from the original on 2017-08-06. Retrieved 5 August 2017.
- plantguy (28 May 2017). "The Selfish Plant 4 – Plant Proprioception?". How Plants Work. Retrieved 5 August 2017.
- Antani, Jyot D.; Gupta, Rachit; Lee, Annie H.; Rhee, Kathy Y.; Manson, Michael D.; Lele, Pushkar P. (2021-09-14). "Mechanosensitive recruitment of stator units promotes binding of the response regulator CheY-P to the flagellar motor". Nature Communications. 12 (1): 5442. Bibcode:2021NatCo..12.5442A. doi:10.1038/s41467-021-25774-2. ISSN 2041-1723. PMC 8440544. PMID 34521846.
External links
- Proprioception at the US National Library of Medicine Medical Subject Headings (MeSH)