Rust (fungus)
Rusts are plant diseases caused by pathogenic fungi of the order Pucciniales (previously known as Uredinales).
| Rusts | |
|---|---|
.jpg.webp) | |
| Puccinia recondita f.sp. tritici on wheat leaf | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Basidiomycota |
| Class: | Pucciniomycetes |
| Order: | Pucciniales |
| Families | |
| |
An estimated 168 rust genera and approximately 7,000 species, more than half of which belong to the genus Puccinia, are currently accepted.[2] Rust fungi are highly specialized plant pathogens with several unique features. Taken as a group, rust fungi are diverse and affect many kinds of plants. However, each species has a very narrow range of hosts and cannot be transmitted to non-host plants. In addition, most rust fungi cannot be grown easily in pure culture.
A single species of rust fungi may be able to infect two different plant hosts in different stages of its life cycle, and may produce up to five morphologically and cytologically distinct spore-producing structures viz., spermogonia, aecia, uredinia, telia, and basidia in successive stages of reproduction.[3] Each spore type is very host specific, and can typically infect only one kind of plant.
Rust fungi are obligate plant pathogens that only infect living plants. Infections begin when a spore lands on the plant surface, germinates, and invades its host. Infection is limited to plant parts such as leaves, petioles, tender shoots, stem, fruits, etc.[2] Plants with severe rust infection may appear stunted, chlorotic (yellowed), or may display signs of infection such as rust fruiting bodies. Rust fungi grow intracellularly, and make spore-producing fruiting bodies within or, more often, on the surfaces of affected plant parts.[2] Some rust species form perennial systemic infections that may cause plant deformities such as growth retardation, witch's broom, stem canker, galls, or hypertrophy of affected plant parts.
Rusts get their name because they are most commonly observed as deposits of powdery rust-coloured or brown spores on plant surfaces. The Roman agricultural festival Robigalia (April 25) has ancient origins in combating wheat rust.[4]
Impacts
Rusts are considered among the most harmful pathogens to agriculture, horticulture and forestry. Rust fungi are major concerns and limiting factors for successful cultivation of agricultural and forest crops. White pine blister rust, wheat stem rust, soybean rust, and coffee rust are examples of notoriously damaging threats to economically important crops.[2] Climate change can have a possible impact to increase rust fungi by increase in CO2 and O3, climate warming, humidity, extreme weather changes.[5]
Life cycle
All rusts are obligate parasites, meaning that they require a living host to complete their life cycle. They generally do not kill the host plant but can severely reduce growth and yield.[6] Cereal crops can be devastated in one season; oak trees infected in the main stem within their first five years by the rust Cronartium quercuum often die.[7]
_-Osterloh-_-Brendel_10_h%252C_2-.jpg.webp)
Rust fungi can produce up to five spore types from corresponding fruiting body types during their life cycle, depending on the species. Roman numerals have traditionally been used to refer to these morphological types.
- 0-Pycniospores (Spermatia) from Pycnidia. These serve mainly as haploid gametes in heterothallic rusts.
- I-Aeciospores from Aecia. These serve mainly as non-repeating, dikaryotic, asexual spores, and go on to infect the primary host.
- II-Urediniospores from Uredia (Uredinia). These serve as repeating dikaryotic vegetative spores. These spores are referred to as the repeating stage because they can cause auto-infection on the primary host, re-infecting the same host on which the spores were produced. They are often profuse, red/orange, and a prominent sign of rust disease.
- III-Teliospores from Telia. These dikaryotic spores are often the survival/overwintering stage of the life cycle. They usually do not infect a plant directly; instead they germinate to produce basidia and basidiospores.
- IV-Basidiospores from Teliospores. These windborne haploid spores often infect the alternate host in Spring.[8][9] They are rarely observed outside of the laboratory.
Rust fungi are often categorized by their life cycle. Three basic types of life cycles are recognized based on the number of spore types as macrocyclic, demicyclic, and microcyclic.[2] The macrocyclic life cycle has all spore states, the demicyclic lacks the uredinial state, and the microcyclic cycle lacks the basidial, pycnial, and the aecial states, thus possess only uredinia and telia. Spermagonia may be absent from each type but especially the microcyclic life cycle. In macrocyclic and demicyclic life cycles, the rust may be either host alternating (heteroecious) (i.e., the aecial state is on one kind of plant but the telial state on a different and unrelated plant), or non-host alternating (autoecious) (i.e., the aecial and telial states on the same plant host).[2] Heteroecious rust fungi require two unrelated hosts to complete their life cycle, with the primary host being infected by aeciospores and the alternate host being infected by basidiospores. This can be contrasted with an autoecious fungus, such as Puccinia porri, which can complete all parts of its life cycle on a single host species.[8] Understanding the life cycles of rust fungi allows for proper disease management.[10]
Host plant-rust fungus relationship
There are definite patterns of relationship with host plant groups and the rust fungi that parasitize them. Some genera of rust fungi, especially Puccinia and Uromyces, comprise species that are capable of parasitizing plants of many families. Other rust genera appear to be restricted to certain plant groups. Host restriction may, in heteroecious species, apply to both phases of life cycle or to only one phase.[2] As with many pathogen/host pairs, rusts are often in gene-for-gene relationships with their plants. This rust-plant gene-for-gene interaction differs somewhat from other gene-for-gene situations and has its own quirks and agronomic significance.
Infection process
The spores of rust fungi may be dispersed by wind, water or insect vectors.[11] When a spore encounters a susceptible plant, it can germinate and infect plant tissues. A rust spores typically germinates on a plant surface, growing a short hypha called a germ tube. This germ tube may locate a stoma by a touch responsive process known as thigmotropism. This involves orienting to ridges created by epidermal cells on the leaf surface, and growing directionally until it encounters a stoma.[12]

Over the stoma, a hyphal tip produces an infection structure called an appressorium. From the underside of an appressorium, a slender hypha grows downward to infect plant cells[13] It is thought that the whole process is mediated by stretch-sensitive calcium ion channels located in the tip of the hypha, which produce electric currents and alter gene expression, inducing appressorium formation.[14]
Once the fungus has invaded the plant, it grows into plant mesophyll cells, producing specialized hyphae known as haustoria. The haustoria penetrate cell walls but not cell membrances: plant cell membranes invaginate around the main haustorial body forming a space known as the extra-haustorial matrix. An iron and phosphorus rich neck band bridges the plant and fungal membranes in the space between the cells for water flow, known as the apoplast, thus preventing the nutrients reaching the plant's cells. The haustorium contains amino acid and hexose sugar transporters and H+-ATPases which are used for active transport of nutrients from the plant, nourishing the fungus.[15] The fungus continues growing, penetrating more and more plant cells, until spore growth occurs. The process repeats every 10 – 14 days, producing numerous spores that can be spread to other parts of the same plant, or to new hosts.
Common rust fungi in agriculture
- Cronartium ribicola (White pine blister rust); the primary hosts are currants, and white pines the secondary. Heterocyclic and macrocyclic
- Gymnosporangium juniperi-virginianae (Cedar-apple rust); Juniperus virginiana is the primary (telial) host and apple, pear or hawthorn is the secondary (aecial) host. Heteroecious and demicyclic
- Hemileia vastatrix (Coffee rust); Primary host is coffee plant; unknown alternate host. Heteroecious
- Phakopsora meibomiae and P. pachyrhizi (Soybean rust); Primary host is soybean and various legumes. Unknown alternate host. Heteroecious
- Puccinia coronata (Crown Rust of Oats and Ryegrass); Oats are the primary host; Rhamnus spp. (Buckthorn) is alternate host. Heteroecious and macrocyclic
- Puccinia graminis (Stem rust of wheat and Kentucky bluegrass, or black rust of cereals); Primary hosts include: Kentucky bluegrass, barley, and wheat; Common barberry is the alternate host. Heteroecious and macrocyclic
- Puccinia hemerocallidis (Daylily rust); Daylily is primary host; Patrinia sp is alternate host. Heteroecious and macrocyclic
- Puccinia triticina (Brown Wheat Rust) in grains
- Puccinia sorghi (Common Rust of Corn)[17]
- Puccinia striiformis (Yellow Rust) of cereals
- Uromyces appendiculatus (Bean Rust) in common bean (Phaseolus vulgaris)[18]
- Puccinia melanocephala (Brown Rust of Sugarcane)
- Puccinia kuehnii (Orange rust of Sugarcane)
- Puccinia porri (Leek rust); Autoecious
Management of rust fungi diseases
Research
Efforts to control rusts began to be scientifically based in the 20th century.[19] Elvin C. Stakman initiated the scientific study of host resistance, which had heretofore been poorly understood and handled by individual growers as part of the breeding process.[19] Stakman was followed by H. H. Flor's extensive discoveries of rust genetics.[19] In order to study rust metabolics, Tervet et al., 1951 developed the cyclone separator.[19] The cyclone separator allows the mechanised collection of spores for study – Cherry & Peet 1966's improved version gathers even more efficiently.[19] (Cherry & Peet 1966 also resulted in the US government's abandonment of all biological weapons programs using rust fungi.)[19] This device was first put to work testing the composition of the spores themselves, especially substances coating the outside of the spores which signal population density.[19] When detected they help prevent crowding.[19]
Control
The control methods of rust fungus diseases depend largely on the life cycle of the particular pathogen. The following are examples of disease management plans used to control macrocyclic and demicyclic diseases:
Macrocyclic Disease: Developing a management plan for this type of disease depends largely on whether the repeating stage (urediniospores) occur on the economically important host plant or the alternate host. For example, the repeating stage in white pine blister rust disease does not occur on white pines but on the alternate host, Ribes spp. During August and September Ribes spp. give rise to teliospores which infect white pines. Removal of the alternate host disrupts the life cycle of the rust fungi Cronartium ribicola, preventing the formation of basidiospores which infect the primary host. Although spores from white pines cannot infect other white pines, survival spores may overwinter on infected pines and reinfect Ribes spp. the following season. Infected tissue is removed from white pines and strict quarantines of Ribes spp. are maintained in high risk areas.[8][20]
Puccinia graminis is a macrocyclic heteroecious fungus that causes wheat stem rust disease. The repeating stage in this fungus occurs on wheat and not the alternate host, barberry. The repeating stage allows the disease to persist in wheat even though the alternate host may be removed. Planting resistant crops is the ideal form of disease prevention, however, mutations can give rise to new strains of fungi that can overcome plant resistance. Although the disease cannot be stopped by removal of the alternate host, the life cycle is disrupted and the rate of mutation is decreased because of reduced genetic recombination. This allows resistance bred crops to remain effective for a longer period of time.[8][21]
Demicyclic Disease: Because there is no repeating stage in the life cycle of demicyclic fungi, removal of the primary or the alternate host will disrupt the disease cycle. This method, however, is not highly effective in managing all demicyclic diseases. Cedar-apple rust disease, for example, can persist despite removal of one of the hosts since spores can be disseminated from long distances. The severity of Cedar-apple rust disease can be managed by removal of basidiospore producing galls from junipers or the application of protective fungicides to junipers.[22]
Home control
Rust diseases are very hard to treat. Fungicides such as Mancozeb or Triforine may help but may never eradicate the disease. Some organic preventative solutions are available and sulphur powder is known to stop spore germination. High standards of hygiene, good soil drainage, and careful watering may minimize problems. Any appearance of rust must be immediately dealt with by removing and burning all affected leaves. Composting, or leaving infected vegetation on the ground will spread the disease.
Commercial control
In some large acreage crops, fungicides are applied by air. The process is expensive and fungicide application is best reserved for seasons when foliar diseases are severe. Research indicates, the higher the foliar disease severity, the greater the return from the use of fungicides.[23] Southern corn rust disease, can be confused with common rust. Southern rust's distinguishing characteristic is that pustules form mostly on the upper leaf surface and spores are more orange in color. Southern rust spreads more quickly and has a higher economic impact when hot, humid weather conditions persist. Timely fungicide applications to control southern rust are more crucial than with common rust.[24]
A variety of preventative methods can be employed for rust diseases:
- High moisture levels may exacerbate rust disease symptoms. The avoidance of overhead watering at night, using drip irrigation, reducing crop density, and using fans to circulate air flow may decrease disease severity.
- The use of rust resistant plant varieties
- Crop rotation can break the disease cycle because many rusts are host specific and do not persist long without their host.
- Inspection of imported plants and cuttings for symptoms. It is important to continuously observe the plants because rust diseases have a latent period (plant has the disease but shows no symptoms).
- Use of disease-free seed can reduce incidence for some rusts[21]
Host plants affected
It is probable that most plant species are affected by some species of rust. Rusts are often named after a host species that they infect. For example; Puccinia xanthii infects the flowering plant cocklebur (Xanthium). Recently, a total of 95 rust fungi belonging to 25 genera associated with 117 forest plant species belonging to 80 host genera under 43 host families were reported from the Western Ghats, Kerala, India.[2] Rust fungi include:
- Aecidium
- Cerotelium
- Chaconia
- Coleosporium
- Crossopsora
- Didymopsorella
- Hamspora
- Hapalophragmidium
- Hemileia
- Kernkampella
- Kuehneola
- Kweilingia
- Macabuna
- Maravalia
- Melampsora
- Olivea
- Physopella
- Puccinia
- Ravenelia
- Uraecium
- Uredo
- Uredopeltis
- Uromyces
- Xenostele
- Zaghouania
Rust infected host genera include:[2]
- Acacia
- Acalypha
- Ageratina
- Albizia
- Arundinaria
- Bambusa
- Bidens
- Blepharis
- Bombax
- Bridelia
- Callicarpa
- Canarium
- Canthium
- Catunaragam
- Cinnamomum
- Cissus
- Cleistanthus
- Clerodendron
- Coffea
- Coix
- Cosmostigma
- Crotalaria
- Dalbergia
- Dendrocalamus
- Derris
- Diospyros
- Dipterocanthus
- Elaeagnus
- Elephantopus
- Elettaria
- Eragrostis
- Euphorbia
- Ficus
- Flacourtia
- Grewia
- Holarrhena
- Holoptelia
- Hypericum
- Ichnocarpus
- Ischaemum
- Jasminum
- Justicia
- Loesneriella
- Luvunga
- Meiogyne
- Meliosma
- Mimusops
- Morus
- Neolitzea
- Ocimum
- Olea
- Oxalis
- Pavetta
- Persicaria
- Phyllanthus
- Plectranthus
- Plumeria
- Pongamia
- Premna
- Protasparagus
- Rubus
- Salix
- Spondia
- Strobilanthes
- Strychnos
- Tabernaemontana
- Terminalia
- Toddalia
- Trichosanthes
- Vernonia
- Vigna
- Wrightia
- Xanthophyllum
- Xylia
- Ziziphus
Some of the better known hosts include:
- Arisaema triphyllum, Jack-in-the-pulpit
- Avena sativa, Oats
- Berberis vulgaris, Common barberry
- Vicia faba - Broad beans
- Coffea arabica - Coffee
- Crataegus monogyna - Hawthorn
- Chrysanthemum
- Cydonia - Quince
- Euphorbia maculata, Spotted Spurge
- Fuchsia spp,
- Garlic
- Hordeum vulgare, Barley
- Juniperus virginiana, Red Cedar (Juniper apple disease)
- Juniperus communis - Juniper
- Allium ampeloprasum - Leek
- Malus – Apple
- Mentha piperita - Peppermint
- Mespilus - Medlar
- Onion
- Pelargonium
- Primula veris
- Primula vulgaris
- Pyrus - Pear
- Rosa spp, Roses
- Triticum spp., Wheat
- Oxalis spp., Oxalis
- Secale cereale, Rye
- Senecio vulgaris -Common groundsel
- Xanthium canadense Cocklebur
Hyperparasites of rusts
In the family Sphaeropsidaceae of Sphaeropsidales fungi, species of the genus Darluca are hyperparasites on rusts.[25]
Gallery
_Pengo.jpg.webp) Rust fungus on a leaf, under low magnification.
Rust fungus on a leaf, under low magnification._pustules_of_urediniospores_Pengo.jpg.webp) Urediniospores of a rust fungus.
Urediniospores of a rust fungus.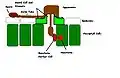 Diagram representing the infection process of rust fungi
Diagram representing the infection process of rust fungi Rust fungus, Puccinia urticata on the surface of a nettle leaf
Rust fungus, Puccinia urticata on the surface of a nettle leaf Rust on onions
Rust on onions
See also
- Stem rust
- Wheat leaf rust
- Leaf rust (barley)
- Fungus
- Smut (fungus)
- Soybean rust
- Rust (programming language) (named after the Rust fungus)
References
- "Species Fungorum - Search Page". www.speciesfungorum.org. Retrieved 27 October 2022.
- Mohanan C. (2010). Rust Fungi of Kerala. Kerala, India: Kerala Forest Research Institute. p. 148. ISBN 978-81-85041-72-8.
- Kolmer, James A; Ordonez, Maria E; Groth, James V (2001). eLS. John Wiley & Sons, Ltd. doi:10.1002/9780470015902.a0021264. ISBN 9780470015902.
- Evans, R. (2007). Utopia Antiqua: Readings of the Golden Age and Decline at Rome. Taylor & Francis. ISBN 978-1-134-48787-5. Retrieved 2018-01-12.
- Helfer, Stephan (2013-10-30). "Rust fungi and global change". New Phytologist. 201 (3): 770–780. doi:10.1111/nph.12570. ISSN 0028-646X.
- Central Science Laboratory. (2006). Plant Healthcare: Rusts [Fact Sheet]. Retrieved from www.csldiagnostics.co.uk
- "Rust Fungi". www.backyardnature.net.
- Schumann, G. & D'Arcy, C. (2010). Essential plant pathology. APS Press
- Scott, K.J, & Chakravorty, A.K., (1982), The Rust fungi. Academic Press.
- Peterson, R., (1974). The Rust Fungus Life Cycle. The Botanical Review. 40(4), 453-513.
- Craigie, J.H. (1931). Phytopathology, 21,1001
- Dickinson, M. Molecular Plant Pathology. 2003.
- Deising, H.B., S. Werner, and M. Wernitz, The role of fungal appressoria in plant infection. Microbes Infect, 2000. 2(13): p. 1631-41.
- Zhou, X.L., et al., A mechanosensitive channel in whole cells and in membrane patches of the fungus Uromyces. Science, 1991. 253(5026): p. 1415.
- Voegele, R.T. and K. Mendgen, Rust haustoria: nutrient uptake and beyond. New Phytologist, 2003. 159(1): p. 93-100.
- Cornell University. (2010). Daylily rust: Puccinia hemerocallidis [Fact sheet]. Retrieved from http://plantclinic.cornell.edu
- Hooker, Arthur L (1967). "The Genetics and Expression of Resistance in Plants to Rusts of the Genus Puccinia". Annu. Rev. Phytopathol. 5 (1): 163–178. doi:10.1146/annurev.py.05.090167.001115.
- Hurtado-Gonzales, O. P.; Valentini, G.; Gilio, T. A.; Martins, A. M.; Song, Q.; Pastor-Corrales, M. A. (2016). "Fine Mapping of Ur-3, a Historically Important Rust Resistance Locus in Common Bean". G3: Genes, Genomes, Genetics. 7 (2): 557–569. doi:10.1534/g3.116.036061. PMC 5295601. PMID 28031244.
- Staples, Richard (2000). "Research on the Rust Fungi During the Twentieth Century". Annual Review of Phytopathology. Annual Reviews. 38 (1): 49–69. doi:10.1146/annurev.phyto.38.1.49. ISSN 0066-4286. PMID 11701836. S2CID 4861612.
- Cornell University. (2005). White Pine Blister Rust: Cronartium ribicola [Fact sheet]. Retrieved from http://plantclinic.cornell.edu
- Marsalis, M. & Goldberg, N. (2006). Leaf, Stem, And Stripe Rust Diseases of Wheat. [Fact sheet]. New Mexico State University
- Wallis, C. & Lewandowski, D. (2008). Cedar Rust Diseases of Ornamental Plants. [Fact Sheet]. Ohio State University
- "Stopsoybeanrust.com". www.stopsoybeanrust.com.
- "Common Corn Rust". www.channel.com.
- faculty.ucr.edu (retrieved December 2015)
External links
 Media related to Pucciniales at Wikimedia Commons
Media related to Pucciniales at Wikimedia Commons