Crinoid
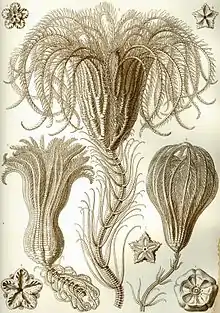
Crinoids are marine animals that make up the class Crinoidea, one of the classes of the phylum Echinodermata, which also includes the starfish, brittle stars, sea urchins and sea cucumbers.[3] Those crinoids which, in their adult form, are attached to the sea bottom by a stalk are commonly called sea lilies, while the unstalked forms are called feather stars or comatulids, being members of the largest crinoid order, Comatulida. They live in both shallow water[4] and in depths as great as 9,000 meters (30,000 ft).[5]
| Crinoids Temporal range: | |
|---|---|
 | |
| Crinoid on the reef of Batu Moncho Island, Indonesia | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Echinodermata |
| Subphylum: | Crinozoa |
| Class: | Crinoidea Miller, 1821[2] |
| Major groups | |
| |
Adult crinoids are characterised by having the mouth located on the upper surface. This is surrounded by feeding arms, and is linked to a U-shaped gut, with the anus being located on the oral disc near the mouth. Although the basic echinoderm pattern of fivefold symmetry can be recognised, in most crinoids the five arms are subdivided into ten or more. These have feathery pinnules and are spread wide to gather planktonic particles from the water. At some stage in their lives, most crinoids have a stem used to attach themselves to the substrate, but many live attached only as juveniles and become free-swimming as adults.
There are only about 600 living species of crinoid,[6] but the class was much more abundant and diverse in the past. Some thick limestone beds dating to the mid-Paleozoic to Jurassic eras are almost entirely made up of disarticulated crinoid fragments.[7][8][9]
Etymology
The name "Crinoidea" comes from the Ancient Greek word κρίνον (krínon), "a lily", with the suffix –oid meaning "like".[10][11] Those crinoids which in their adult form are attached to the sea bottom by a stalk are commonly called sea lilies,[12] while the unstalked forms are called feather stars[13] or comatulids, being members of the largest crinoid order, Comatulida.[14]
Morphology
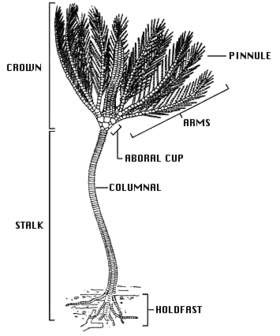
The basic body form of a crinoid is a stem (not present in adult feather stars) and a crown consisting of a cup-like central body known as the theca, and a set of five rays or arms, usually branched and feathery. The mouth and anus are both located on the upper side of the theca, making the dorsal (upper) surface the oral surface, unlike in the other echinoderm groups such as the sea urchins, starfish and brittle stars where the mouth is on the underside.[15] The numerous calcareous plates make up the bulk of the crinoid, with only a small percentage of soft tissue. These ossicles fossilise well and there are beds of limestone dating from the Lower Carboniferous around Clitheroe, England, formed almost exclusively from a diverse fauna of crinoid fossils.[16]
The stem of sea lilies is composed of a column of highly porous ossicles which are connected by ligamentary tissue. It attaches to the substrate with a flattened holdfast or with whorls of jointed, root-like structures known as cirri. Further cirri may occur higher up the stem. In crinoids that attach to hard surfaces, the cirri may be robust and curved, resembling birds' feet, but when crinoids live on soft sediment, the cirri may be slender and rod-like. Juvenile feather stars have a stem, but this is later lost, with many species retaining a few cirri at the base of the crown. The majority of living crinoids are free-swimming and have only a vestigial stalk. In those deep-sea species that still retain a stalk, it may reach up to 1 m (3 ft) in length (although usually much smaller), and fossil species are known with 20 m (66 ft) stems,[17] the largest recorded crinoid having a stem 40 m (130 ft) in length.[18]
The theca is pentamerous (has five-part symmetry) and is homologous with the body or disc of other echinoderms. The base of the theca is formed from a cup-shaped set of ossicles (bony plates), the calyx, while the upper surface is formed by the weakly-calcified tegmen, a membranous disc. The tegmen is divided into five "ambulacral areas", including a deep groove from which the tube feet project, and five "interambulacral areas" between them. The mouth is near the centre or on the margin of the tegmen, and ambulacral grooves lead from the base of the arms to the mouth. The anus is also located on the tegmen, often on a small elevated cone, in an interambulacral area. The theca is relatively small and contains the crinoid's digestive organs.[17]
The arms are supported by a series of articulating ossicles similar to those in the stalk. Primitively, crinoids had only five arms, but in most modern forms these are divided into two at ossicle II, giving ten arms in total. In most living species, especially the free-swimming feather stars, the arms branch several more times, producing up to two hundred branches in total. Being jointed, the arms can curl up. They are lined, on either side alternately, by smaller jointed appendages known as "pinnules" which give them their feather-like appearance. Both arms and pinnules have tube feet along the margins of the ambulacral grooves. The tube feet come in groups of three of different size; they have no suction pads and are used to hold and manipulate food particles. The grooves are equipped with cilia which facilitate feeding by moving the organic particles along the arm and into the mouth.[17]
 Stem, theca and arms of a "true" (stalked) crinoid (family Isselicrinidae)
Stem, theca and arms of a "true" (stalked) crinoid (family Isselicrinidae) Oxycomanthus bennetti (comatulid)
Oxycomanthus bennetti (comatulid) Tegmen of a Lamprometra palmata. The mouth is located at the center of the 5 feeding grooves, and the anus at the top of the column.
Tegmen of a Lamprometra palmata. The mouth is located at the center of the 5 feeding grooves, and the anus at the top of the column. Close-up on the cirri that allow comatulids to walk and attach themselves
Close-up on the cirri that allow comatulids to walk and attach themselves Close-up on the pinnules of a Tropiometra carinata (with parasites Myzostoma fuscomaculatum)
Close-up on the pinnules of a Tropiometra carinata (with parasites Myzostoma fuscomaculatum)
Biology
Feeding

Crinoids are passive suspension feeders, filtering plankton and small particles of detritus from the sea water flowing past them with their feather-like arms. The arms are raised to form a fan-shape which is held perpendicular to the current. Mobile crinoids move to perch on rocks, coral heads or other eminences to maximise their feeding opportunities. The food particles are caught by the primary (longest) tube feet, which are fully extended and held erect from the pinnules, forming a food-trapping mesh, while the secondary and tertiary tube feet are involved in manipulating anything encountered.[17]
The tube feet are covered with sticky mucus that traps any particles which come in contact. Once they have caught a particle of food, the tube feet flick it into the ambulacral groove, where the cilia propel the mucus and food particles towards the mouth. Lappets at the side of the groove help keep the mucus stream in place. The total length of the food-trapping surface may be very large; the 56 arms of a Japanese sea lily with 24 cm (9 in) arms, have a total length of 80 m (260 ft) including the pinnules. Generally speaking, crinoids living in environments with relatively little plankton have longer and more highly branched arms than those living in food-rich environments.[17]
The mouth descends into a short oesophagus. There is no true stomach, so the oesophagus connects directly to the intestine, which runs in a single loop right around the inside of the calyx. The intestine often includes numerous diverticulae, some of which may be long or branched. The end of the intestine opens into a short muscular rectum. This ascends towards the anus, which projects from a small conical protuberance at the edge of the tegmen. Faecal matter is formed into large, mucous-cemented pellets which fall onto the tegmen and thence the substrate.[17]
Predation
Specimens of the sea urchin Calocidaris micans found in the vicinity of the crinoid Endoxocrinus parrae, have been shown to contain large quantities of stem portions in their guts. These consist of articulated ossicles with soft tissue, whereas the local sediment contained only disarticulated ossicles without soft tissue. This makes it highly likely that these sea urchins are predators of the crinoids, and that the crinoids flee, offering part of their stem in the process.[19]
Various crinoid fossils hint at possible prehistoric predators. Coprolites of both fish and cephalopods have been found containing ossicles of various crinoids, such as the pelagic crinoid Saccocoma, from the Jurassic lagerstatten Solnhofen,[20] while damaged crinoid stems with bite marks matching the toothplates of coccosteid placoderms have been found in Late Devonian Poland.[21] The calyxes of several Devonian to Carboniferous-aged crinoids have the shells of a snail, Platyceras, intimately associated with them.[22] Some have the snail situated over the anus, suggesting that Platyceras was a coprophagous commensal, while others have the animal directly situated over a borehole, suggesting a more pernicious relationship.[23]
Water vascular system
Like other echinoderms, crinoids possess a water vascular system that maintains hydraulic pressure in the tube feet. This is not connected to external sea water via a madreporite, as in other echinoderms, but only connected through a large number of pores to the coelom (body cavity). The main fluid reservoir is the muscular-walled ring canal which is connected to the coelom by stone canals lined with calcareous material. The coelom is divided into a number of interconnecting spaces by mesenteries. It surrounds the viscera in the disc and has branches within the stalk and arms, with smaller branches extending into the pinnules. It is the contraction of the ring canal that extends the tube feet. Three narrow branches of the coelom enter each arm, two on the oral side and one aborally, and pinnules. The action of cilia cause there to be a slow flow of fluid (1mm per second) in these canals, outward in the oral branches and inward in the aboral ones, and this is the main means of transport of nutrients and waste products. There is no heart and separate circulatory system but at the base of the disc there is a large blood vessel known as the axial organ, containing some slender blind-ended tubes of unknown function, which extends into the stalk.[17]
These various fluid-filled spaces, in addition to transporting nutrients around the body, also function as both a respiratory and an excretory system. Oxygen is absorbed primarily through the tube feet, which are the most thin-walled parts of the body, with further gas exchange taking place over the large surface area of the arms. There are no specialised organs for excretion while waste is collected by phagocytic coelomocytes.[17]
Nervous system
The crinoid nervous system is divided into three parts, with numerous connections between them. The oral or uppermost portion is the only one homologous with the nervous systems of other echinoderms. It consists of a central nerve ring surrounding the mouth, and radial nerves branching into the arms and is sensory in function. Below this lies an intermediate nerve ring, giving off radial nerves supplying the arms and pinnules. These nerves are motor in nature, and control the musculature of the tube feet. The third portion of the nervous system lies aborally, and is responsible for the flexing and movement actions of the arms, pinnules and cirri. This is centred on a mass of neural tissue near the base of the calyx, and provides a single nerve to each arm and a number of nerves to the stalk.[17]
Reproduction and life cycle
Crinoids are not capable of clonal reproduction as are some starfish and brittle stars, but are capable of regenerating lost body parts. Arms torn off by predators or damaged by adverse environmental conditions can regrow, and even the visceral mass can regenerate over the course of a few weeks. This regeneration may be vital in surviving attacks by predatory fish.[17]
Crinoids are dioecious, with individuals being either male or female. In most species, the gonads are located in the pinnules but in a few, they are located in the arms. Not all the pinnules are reproductive, just those closest to the crown. The gametes are produced in genital canals enclosed in genital coeloms. The pinnules eventually rupture to release the sperm and eggs into the surrounding sea water. In certain genera, such as Antedon, the fertilised eggs are cemented to the arms with secretions from epidermal glands; in others, especially cold water species from Antarctica, the eggs are brooded in specialised sacs on the arms or pinnules.[17]
The fertilised eggs hatch to release free-swimming vitellaria larvae. The bilaterally symmetrical larva is barrel-shaped with rings of cilia running round the body, and a tuft of sensory hairs at the upper pole. While both feeding (planktotrophic) and non-feeding (lecithotrophic) larvae exist among the four other extant echinoderm classes, all present day crinoids appear to be descendants from a surviving clade that went through a bottleneck after the Permian extinction, at that time losing the feeding larval stage.[24] The larva's free-swimming period lasts for only a few days before it settles on the bottom and attaches itself to the underlying surface using an adhesive gland on its underside. The larva then undergoes an extended period of metamorphoses into a stalked juvenile, becoming radially symmetric in the process. Even the free-swimming feather stars go through this stage, with the adult eventually breaking away from the stalk.[17]
Locomotion

Most modern crinoids, i.e., the feather stars, are free-moving and lack a stem as adults. Examples of fossil crinoids that have been interpreted as free-swimming include Marsupites, Saccocoma and Uintacrinus.[25] In general, crinoids move to new locations by crawling, using the cirri as legs. Such a movement may be induced in relation to a change in current direction, the need to climb to an elevated perch to feed, or because of an agonistic behaviour by an encountered individual.[26] Crinoids can also swim. They do this by co-ordinated, repeated sequential movements of the arms in three groups. At first the direction of travel is upwards but soon becomes horizontal, travelling at about 7 cm (2.8 in) per second with the oral surface in front. Swimming usually takes place as short bursts of activity lasting up to half a minute, and in the comatulid Florometra serratissima at least, only takes place after mechanical stimulation or as an escape response evoked by a predator.[26]
In 2005, a stalked crinoid was recorded pulling itself along the sea floor off the Grand Bahama Island. While it has been known that stalked crinoids could move, before this recording the fastest motion known for a stalked crinoid was 0.6 metres (2 feet) per hour. The 2005 recording showed one of these moving across the seabed at the much faster rate of 4 to 5 cm (1.6 to 2.0 in) per second, or 144 to 180 m (472 to 591 ft) per hour.[27]
Evolution
Origins


If one ignores the enigmatic Echmatocrinus of the Burgess Shale, the earliest known unequivocal crinoid groups date back to the Ordovician, 480 million years ago. There are two competing hypotheses pertaining to the origin of the group: the traditional viewpoint holds that crinoids evolved from within the blastozoans (the eocrinoids and their derived descendants, the blastoids and the cystoids), whereas the most popular alternative suggests that the crinoids split early from among the edrioasteroids.[28] The debate is difficult to settle, in part because all three candidate ancestors share many characteristics, including radial symmetry, calcareous plates, and stalked or direct attachment to the substrate.[28]
Diversity
Echinoderms with mineralized skeletons entered the fossil record in the early Cambrian (540 mya), and during the next 100 million years, the crinoids and blastoids (also stalked filter-feeders) were dominant.[29] At that time, the Echinodermata included twenty taxa of class rank, only five of which survived the mass extinction events that followed. The long and varied geological history of the crinoids demonstrates how well the echinoderms had adapted to filter-feeding.[3]
The crinoids underwent two periods of abrupt adaptive radiation, the first during the Ordovician (485 to 444 mya), and the other during the early Triassic (around 230 mya).[30] This Triassic radiation resulted in forms possessing flexible arms becoming widespread; motility, predominantly a response to predation pressure, also became far more prevalent than sessility.[31] This radiation occurred somewhat earlier than the Mesozoic marine revolution, possibly because it was mainly prompted by increases in benthic predation, specifically of echinoids.[32] There then followed a selective mass extinction at the end of the Permian period, during which all blastoids and most crinoids became extinct.[30] After the end-Permian extinction, crinoids never regained the morphological diversity and dominant position they enjoyed in the Paleozoic; they employed a different suite of ecological strategies open to them from those that had proven so successful in the Paleozoic.[30]
Fossils
Some fossil crinoids, such as Pentacrinites, seem to have lived attached to floating driftwood and complete colonies are often found. Sometimes this driftwood would become waterlogged and sink to the bottom, taking the attached crinoids with it. The stem of Pentacrinites can be several metres long. Modern relatives of Pentacrinites live in gentle currents attached to rocks by the end of their stem. The largest fossil crinoid on record had a stem 40 m (130 ft) in length.[33]
In 2012, three geologists reported they had isolated complex organic molecules from 340-million-year-old (Mississippian) fossils of multiple species of crinoids. Identified as "resembl[ing ...] aromatic or polyaromatic quinones", these are the oldest molecules to be definitively associated with particular individual fossils, as they are believed to have been sealed inside ossicle pores by precipitated calcite during the fossilization process.[34]
Crinoid fossils, and in particular disarticulated crinoid columnals, can be so abundant that they at times serve as the primary supporting clasts in sedimentary rocks. Rocks of this nature are called encrinites.
Taxonomy


Crinoidea has been accepted as a distinct clade of echinoderms since the definition of the group by Miller in 1821.[35] It includes many extinct orders as well as four closely-related living orders (Comatulida, Cyrtocrinida, Hyocrinida, and Isocrinida), which are part of the subgroup Articulata. Living articulates comrpise around 540 species.
- Class Crinoidea
- †Protocrinoidea (incertae sedis)
- Subclass †Camerata
- Order †Diplobathrida
- Order †Monobathrida
- Subclass Pentacrinoidea
- Parvclass †Disparida
- Order †Eustenocrinida
- Order †Maennilicrinida
- Order †Tetragonocrinida
- Order †Calceocrinida
- Parvclass Cladida
- Superorder †Porocrinoidea
- Order †Hybocrinida
- Order †Porocrinida
- Superorder †Flexibilia
- Order †Sagenocrinida
- Order †Taxocrinida
- Magnorder Eucladida
- †Ampelocrinida (incertae sedis)
- Superorder †Cyathoformes
- Superorder Articulata
- Order †Encrinida
- Order †Holocrinida
- Order †Millericrinida
- Order †Roveacrinida
- Order †Uintacrinida
- Order Comatulida
- Order Cyrtocrinida
- Order Hyocrinida
- Order Isocrinida
- Superorder †Porocrinoidea
- Parvclass †Disparida
Phylogeny
The phylogeny, geologic history, and classification of the Crinoidea was discussed by Wright et al. (2017).[36] These authors presented new phylogeny-based and rank-based classifications based on results of recent phylogenetic analyses.[35][37][38][39] Their rank-based classification of crinoid higher taxa (down to Order), not fully resolved and with numerous groups incertae sedis (of uncertain placement), is illustrated in the cladogram.
| Crinoidea |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In culture
Fossilised crinoid columnal segments extracted from limestone quarried on Lindisfarne, or found washed up along the foreshore, were threaded into necklaces or rosaries, and became known as St. Cuthbert's beads in the Middle Ages.[40] Similarly, in the Midwestern United States, fossilized segments of the columns of crinoids are sometimes known as Indian beads.[41] Crinoids are the state fossil of Missouri.[42]
Fossil crinoids
 Fossil from Germany showing the stem, calyx, and arms with pinnules
Fossil from Germany showing the stem, calyx, and arms with pinnules 330 million year old crinoid fossils from Iowa
330 million year old crinoid fossils from Iowa
.jpg.webp) Seirocrinus subangularis from the Early Jurassic Posidonia Shale at Holzmaden, Germany
Seirocrinus subangularis from the Early Jurassic Posidonia Shale at Holzmaden, Germany
 Root-like crinoid holdfast from the Upper Ordovician, southern Ohio
Root-like crinoid holdfast from the Upper Ordovician, southern Ohio Internal mold of crinoid stem lumen (and external mold of stem) from Lower Carboniferous, Ohio
Internal mold of crinoid stem lumen (and external mold of stem) from Lower Carboniferous, Ohio Fossils of Seirocrinus subsingularis from the Jurassic Holzmaden Black Shale Formation, Germany
Fossils of Seirocrinus subsingularis from the Jurassic Holzmaden Black Shale Formation, Germany
References
- Zamora, Samuel; Rahman, Imran A.; Ausich, William I. (2015). "Palaeogeographic implications of a new iocrinid crinoid (Disparida) from the Ordovician (Darriwillian) of Morocco". PeerJ. 3: e1450. doi:10.7717/peerj.1450. PMC 4675106. PMID 26664800.
- Hansson, Hans (2012). "Crinoidea". WoRMS. World Register of Marine Species. Retrieved 2013-01-30.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 917–918. ISBN 978-81-315-0104-7.
- Zmarzly, D.L. (1985). "The Shallow-Water Crinoid Fauna of Kwajalein Atoll, Marshall Islands: Ecological Observations, Interatoll Comparisons, and Zoogeographic Affinities". Pacific Science. 39: 340–358. hdl:10125/941.
- Oji, T.; Ogawa, Y.; Hunter, A. W. & Kitazawa, K. (2009). "Discovery of Dense Aggregations of Stalked Crinoids in Izu-Ogasawara Trench, Japan". Zoological Science. 26 (6): 406–408. doi:10.2108/zsj.26.406. PMID 19583499. S2CID 5991969.
- "Animal Diversity Web: Crinoidea". University of Michigan Museum of Zoology. Retrieved 26 August 2012.
- Lucia, F. Jerry (1962). "Diagenesis of a Crinoidal Sediment". SEPM Journal of Sedimentary Research. 32: 848–865. doi:10.1306/74D70D8F-2B21-11D7-8648000102C1865D.
- Blyth Cain, J. D. (September 1968). "Aspects of the depositional environment and palaeoecology of crinoidal limestones". Scottish Journal of Geology. 4 (3): 191–208. doi:10.1144/sjg04030191. S2CID 219538295.
- Jach, Renata (April 2005). "Storm-dominated deposition of the Lower Jurassic crinoidal limestones in the Krížna unit, Western Tatra Mountains, Poland". Facies. 50 (3–4): 561–572. doi:10.1007/s10347-004-0028-3. S2CID 128947091.
- Webster's New Universal Unabridged Dictionary. 2nd ed. 1979.
- "crinoid". Online Etymology Dictionary.
- "Sea lily". Encyclopædia Britannica. Retrieved 14 March 2011.
- "Feather star". Encyclopædia Britannica. Retrieved 14 March 2011.
- Ausich, William I.; Messing, Charles G. "Crinoidea". Tree of Life. Retrieved 14 March 2011.
- O'Hara, Timothy; Byrne, Maria (2017). Australian Echinoderms: Biology, Ecology and Evolution. Csiro Publishing. pp. 171–180. ISBN 978-1-4863-0763-0.
- Hess, Hans; Brett, Carlton E.; Ausich, William I.; Simms, Michael J. (2002). Fossil Crinoids. Cambridge University Press. pp. 3–5, 45–46. ISBN 978-0-521-52440-7.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 917–927. ISBN 978-81-315-0104-7.
- Ponsonby, David; Dussart, George (2005). The Anatomy of the Sea. Vancouver: Raincoast Books. p. 129. ISBN 978-0-8118-4633-2.
- Baumiller, Tomasz K.; Mooi, Rich; Messing, Charles G. (2008). "Urchins in the meadow: Paleobiological and evolutionary implications of cidaroid predation on crinoids". Paleobiology. 34 (1): 22–34. doi:10.1666/07031.1. JSTOR 20445573. S2CID 85647638.
- Hess, Hans (2003). "Upper Jurassic Solnhofen Plattenkalk of Bavaria, German". In Brett, Carlton E.; Ausich, William I.; Simms, Michael J. (eds.). Fossil Crinoids. Cambridge University Press. pp. 216–24. ISBN 978-0-521-52440-7.
- Gorzelak, Przemys Law; Rakowicz, Lukasz; Salamon, Mariusz A.; Szrek, Piotr (2011). "Inferred placoderm bite marks on Devonian crinoids from Poland". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 259: 105–12. doi:10.1127/0077-7749/2010/0111.
- Brett, Carlton E.; Walker, Sally E. (2002). "Predators and predation in Paleozoic marine environments" (PDF). Paleontological Society Papers. 8: 93–118. doi:10.1017/S1089332600001078. Archived from the original (PDF) on 2012-08-13. Retrieved 2014-04-06.
- Gahn, Forest J.; Baumiller, Tomasz K. (2003). "Infestation of Middle Devonian (Givetian) camerate crinoids by platyceratid gastropods and its implications for the nature of their biotic interaction" (PDF). Lethaia. 36 (2): 71–82. doi:10.1080/00241160310003072. hdl:2027.42/75509.
- Raff, R A; Byrne, M (2006). "The active evolutionary lives of echinoderm larvae". Heredity. 97 (3): 244–52. doi:10.1038/sj.hdy.6800866. PMID 16850040.
- "About Crinoids". FossilEra. Retrieved 15 March 2019.
- Shaw, G.D.; Fontaine, A.R. (2011). "The locomotion of the comatulid Florometra serratissima (Echinodermata: Crinoidea) and its adaptive significance". Canadian Journal of Zoology. 68 (5): 942–950. doi:10.1139/z90-135.
- Baumiller, Tomasz K.; Messing, Charles G. (6 October 2005). "Crawling In Stalked Crinoids: In Situ Observations, Functional Morphology, and Implications for Paleozoic Taxa". Geological Society of America Abstracts with Programs. Vol. 37. p. 62. Archived from the original on 7 April 2014. Retrieved 6 April 2014.
- Guensburg, Thomas E.; Mooi, Rich; Sprinkle, James; David, Bruno; Lefebvre, Bertrand (2010). "Pelmatozoan arms from the mid-Cambrian of Australia: Bridging the gap between brachioles and brachials? Comment: There is no bridge". Lethaia. 43 (3): 432–440. doi:10.1111/j.1502-3931.2010.00220.x.
- Waggoner, Ben (16 January 1995). "Echinodermata: Fossil Record". Introduction to the Echinodermata. Museum of Paleontology: University of California at Berkeley. Retrieved 30 March 2019.
- Foote, Mike (1999). "Morphological diversity in the evolutionary radiation of Paleozoic and post-Paleozoic crinoids". Paleobiology. 25 (sp1): 1–116. doi:10.1666/0094-8373(1999)25[1:MDITER]2.0.CO;2. ISSN 0094-8373. JSTOR 2666042. S2CID 85586709.
- Baumiller, Tomasz K. (2008). "Crinoid Ecological Morphology". Annual Review of Earth and Planetary Sciences. 36: 221–249. Bibcode:2008AREPS..36..221B. doi:10.1146/annurev.earth.36.031207.124116.
- Baumiller, T. K.; Salamon, M. A.; Gorzelak, P.; Mooi, R.; Messing, C. G.; Gahn, F. J. (2010). "Post-Paleozoic crinoid radiation in response to benthic predation preceded the Mesozoic marine revolution". Proceedings of the National Academy of Sciences. 107 (13): 5893–5896. Bibcode:2010PNAS..107.5893B. doi:10.1073/pnas.0914199107. JSTOR 25665085. PMC 2851891. PMID 20231453. INIST:22572914.
- Ponsonby, David; Dussart, George (2005). The Anatomy of the Sea. Vancouver: Raincoast Books. p. 129. ISBN 978-0-8118-4633-2.
- O'Malley, C. E.; Ausich, W. I.; Chin, Y.-P. (2013). "Isolation and characterization of the earliest taxon-specific organic molecules (Mississippian, Crinoidea)". Geology. 41 (3): 347. Bibcode:2013Geo....41..347O. doi:10.1130/G33792.1. Note that the first sentence of the phys.org article contradicts the paper itself, which reviews several isolations of molecules from particular fossils over the past decade.
- Pam Frost Gorder (Feb 19, 2013). "Ancient fossilized sea creatures yield oldest biomolecules isolated directly from a fossil". Phys.org.
- Ausich, William I.; Kammer, Thomas W.; Rhenberg, Elizabeth C.; Wright, David F. (2015). "Early phylogeny of crinoids within the pelmatozoan clade". Palaeontology. 58 (6): 937–952. doi:10.1111/pala.12204.
- Wright, David F.; Ausich, William I.; Cole, Selina R.; Peter, Mark E.; Rhenberg, Elizabeth C. (2017). "Phylogenetic taxonomy and classification of the Crinoidea (Echinodermata)". Journal of Paleontology. 91 (4): 829–846. doi:10.1017/jpa.2016.142.
- Wright, David F. (2017). "Bayesian estimation of fossil phylogenies and the evolution of early to middle Paleozoic crinoids (Echinodermata)". Journal of Paleontology. 91 (4): 799–814. doi:10.1017/jpa.2016.141.
- Cole, Selina R. (2017). "Phylogeny and morphologic evolution of the Ordovician Camerata (Class Crinoidea, Phylum Echinodermata)". Journal of Paleontology. 91 (4): 815–828. doi:10.1017/jpa.2016.137.
- Rouse, Greg W.; Jermiin, Lars S.; Wilson, Nerida G.; Eeckhaut, Igor; Lanterbecq, Deborah; Oji, Tatsuo; Young, Craig M.; Browning, Teena; Cisternas, Paula; Helgen, Lauren E.; Stuckey, Michelle; Messing, Charles G. (2013). "Fixed, free, and fixed: the fickle phylogeny of extant Crinoidea (Echinodermata) and their Permian-Triassic origin". Molecular Phylogenetics and Evolution. 66 (6): 161–181. doi:10.1016/j.ympev.2012.09.018. PMID 23063883.
- Lane, N. Gary; Ausich, William I. (2001). "The Legend of St Cuthbert's Beads: A Palaeontological and Geological Perspective". Folklore. 112 (1): 65–73. JSTOR 1260865.
- "Identifying Unknown Fossils (by their shape)". Kentucky Geological Survey / University of Kentucky. Retrieved 21 June 2009.
- "Missouri's State Fossil". Office of the Secretary of State, Missouri. Retrieved 31 March 2019.
External links
- Messing, Charles. "Sea Star on a Stick: Introducing Crinoids". Vimeo.
 Media related to Crinoidea at Wikimedia Commons
Media related to Crinoidea at Wikimedia Commons Data related to Crinoid at Wikispecies
Data related to Crinoid at Wikispecies



