Wetland
A wetland is a distinct ecosystem that is flooded or saturated by water, either permanently (for years or decades) or seasonally (for weeks or months). Flooding results in oxygen-free (anoxic) processes prevailing, especially in the soils.[1] The primary factor that distinguishes wetlands from terrestrial land forms or water bodies is the characteristic vegetation of aquatic plants, adapted to the unique anoxic hydric soils.[2] Wetlands are considered among the most biologically diverse of all ecosystems, serving as home to a wide range of plant and animal species. Methods for assessing wetland functions, wetland ecological health, and general wetland condition have been developed for many regions of the world. These methods have contributed to wetland conservation partly by raising public awareness of the functions some wetlands provide.[3]
Wetlands occur naturally on every continent.[4] The water in wetlands is either freshwater, brackish or saltwater.[2] The main wetland types are classified based on the dominant plants and/or the source of the water. For example, marshes are wetlands dominated by emergent vegetation such as reeds, cattails and sedges; swamps are ones dominated by woody vegetation such as trees and shrubs (although reed swamps in Europe are dominated by reeds, not trees). Examples of wetlands classified by their sources of water include tidal wetlands (oceanic tides), estuaries (mixed tidal and river waters), floodplains (excess water from overflowed rivers or lakes), springs, seeps and fens (groundwater discharge out onto the surface), and bogs and vernal ponds (rainfall or meltwater).[1][5] Some wetlands have multiple types of plants and are fed by multiple sources of water, making them difficult to classify. The world's largest wetlands include the Amazon River basin, the West Siberian Plain,[6] the Pantanal in South America,[7] and the Sundarbans in the Ganges-Brahmaputra delta.[8]
Wetlands contribute a number of functions that benefit people. These are called ecosystem services and include water purification, groundwater replenishment, stabilization of shorelines and storm protection, water storage and flood control, processing of carbon (carbon fixation, decomposition and sequestration), other nutrients and pollutants, and support of plants and animals.[9] Wetlands are reservoirs of biodiversity and provide wetland products. According to the UN Millennium Ecosystem Assessment, wetlands are more affected by environmental degradation than any other ecosystem on Earth.[10] Wetlands can be important sources and sinks of carbon, depending on the specific wetland, and thus will play an important role in climate change and need to be considered in attempts to mitigate climate change. However, some wetlands are a significant source of methane emissions and some are also emitters of nitrous oxide.[11][12] Constructed wetlands are designed and built to treat municipal and industrial wastewater as well as to divert stormwater runoff. Constructed wetlands may also play a role in water-sensitive urban design.
Definitions and terminology

Technical definitions

A simplified definition of wetland is "an area of land that is usually saturated with water".[13] More precisely, wetlands are areas where "water covers the soil, or is present either at or near the surface of the soil all year or for varying periods of time during the year, including during the growing season".[14] A patch of land that develops pools of water after a rain storm would not necessarily be considered a "wetland", even though the land is wet. Wetlands have unique characteristics: they are generally distinguished from other water bodies or landforms based on their water level and on the types of plants that live within them. Specifically, wetlands are characterized as having a water table that stands at or near the land surface for a long enough period each year to support aquatic plants.[15][16]
A more concise definition is a community composed of hydric soil and hydrophytes.[1]
Wetlands have also been described as ecotones, providing a transition between dry land and water bodies.[17] Wetlands exist "...at the interface between truly terrestrial ecosystems and aquatic systems, making them inherently different from each other, yet highly dependent on both."[18]
In environmental decision-making, there are subsets of definitions that are agreed upon to make regulatory and policy decisions.
Under the Ramsar international wetland conservation treaty, wetlands are defined as follows:[19]
- Article 1.1: "...wetlands are areas of marsh, fen, peatland or water, whether natural or artificial, permanent or temporary, with water that is static or flowing, fresh, brackish or salt, including areas of marine water the depth of which at low tide does not exceed six meters."
- Article 2.1: "[Wetlands] may incorporate riparian and coastal zones adjacent to the wetlands, and islands or bodies of marine water deeper than six meters at low tide lying within the wetlands."
An ecological definition of a wetland is "an ecosystem that arises when inundation by water produces soils dominated by anaerobic and aerobic processes, which, in turn, forces the biota, particularly rooted plants, to adapt to flooding".[1]
Sometimes a precise legal definition of a wetland is required. The definition used for regulation by the United States government is: ‘The term “wetlands” means those areas that are inundated or saturated by surface or ground water at a frequency and duration to support, and that under normal circumstances do support, a prevalence of vegetation typically adapted for life in saturated soil conditions. Wetlands generally included swamps, marshes, bogs, and similar areas.’[20]
For each of these definitions and others, regardless of the purpose, hydrology is emphasized (shallow waters, water-logged soils). The soil characteristics and the plants and animals controlled by the wetland hydrology are often additional components of the definitions.[21]
Types
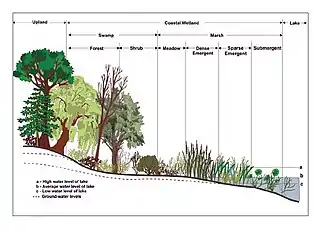
Wetlands can be tidal (inundated by tides) or non-tidal.[22] The water in wetlands is either freshwater, brackish, or saltwater.[2] There are four main kinds of wetlands – marsh, swamp, bog and fen (bogs and fens being types of peatlands or mires). Some experts also recognize wet meadows and aquatic ecosystems as additional wetland types.[1] Sub-types include mangrove forests, carrs, pocosins, floodplains,[1] peatlands, vernal pools, sinks, and many others.[23]
The following three groups are used within Australia to classify wetland by type: Marine and coastal zone wetlands, inland wetlands and human-made wetlands.[24] In the US, the best known classifications are the Cowardin classification system[25] and the hydrogeomorphic (HGM) classification system. The Cowardin system includes five main types of wetlands: marine (ocean-associated); estuarine (mixed ocean- and river-associated); riverine (within river channels); lacustrine (lake-associated); and palustrine (inland nontidal habitats).
Peatlands
Peatlands are a unique kind of wetland where lush plant growth and slow decay of dead plants (under anoxic conditions) results in organic peat accumulating; bogs, fens, and mires are different names for peatlands.
Wetland names
Variations of names for wetland systems:
Some wetlands have localized names unique to a region such as the prairie potholes of North America's northern plain, pocosins, Carolina bays and baygalls[26][27] of the Southeastern US, mallines of Argentina, Mediterranean seasonal ponds of Europe and California, turloughs of Ireland, billabongs of Australia, among many others.
By temperature zone

Wetlands are found throughout the world in different climates.[14] Temperatures vary greatly depending on the location of the wetland. Many of the world's wetlands are in the temperate zones, midway between the North or South Poles and the equator. In these zones, summers are warm and winters are cold, but temperatures are not extreme. In subtropical zone wetlands, such as along the Gulf of Mexico, average temperatures might be 11 °C (52 °F). Wetlands in the tropics are subjected to much higher temperatures for a large portion of the year. Temperatures for wetlands on the Arabian Peninsula can exceed 50 °C (122 °F) and these habitats would therefore be subject to rapid evaporation. In northeastern Siberia, which has a polar climate, wetland temperatures can be as low as −50 °C (−58 °F). Peatlands in arctic and subarctic regions insulate the permafrost, thus delaying or preventing its thawing during summer, as well as inducing its formation.[28]
By precipitation amount
The amount of precipitation a wetland receives varies widely according to its area. Wetlands in Wales, Scotland, and western Ireland typically receive about 1,500 mm (59 in) per year. In some places in Southeast Asia, where heavy rains occur, they can receive up to 10,000 mm (390 in). In some drier regions, wetlands exist where as little as 180 mm (7.1 in) precipitation occurs each year.
Temporal variation:[29]
- Perennial systems
- Seasonal systems
- Episodic (periodic or intermittent) systems
- Ephemeral (short-lived) systems
Surface flow may occur in some segments, with subsurface flow in other segments.
Processes
Wetlands vary widely due to local and regional differences in topography, hydrology, vegetation, and other factors, including human involvement. Other important factors include fertility, natural disturbance, competition, herbivory, burial and salinity.[1] When peat accumulates, bogs and fens arise.
Hydrology
The most important factor producing wetlands is hydrology, or flooding. The duration of flooding or prolonged soil saturation by groundwater determines whether the resulting wetland has aquatic, marsh or swamp vegetation. Other important factors include soil fertility, natural disturbance, competition, herbivory, burial, and salinity.[1] When peat from dead plants accumulates, bogs and fens develop.
Wetland hydrology is associated with the spatial and temporal dispersion, flow, and physio-chemical attributes of surface and ground waters. Sources of hydrological flows into wetlands are predominantly precipitation, surface water (saltwater or freshwater), and groundwater. Water flows out of wetlands by evapotranspiration, surface flows and tides, and subsurface water outflow. Hydrodynamics (the movement of water through and from a wetland) affects hydro-periods (temporal fluctuations in water levels) by controlling the water balance and water storage within a wetland.[30]
Landscape characteristics control wetland hydrology and water chemistry. The O2 and CO2 concentrations of water depend on temperature, atmospheric pressure and mixing with the air (from winds or water flows). Water chemistry within wetlands is determined by the pH, salinity, nutrients, conductivity, soil composition, hardness, and the sources of water. Water chemistry varies across landscapes and climatic regions. Wetlands are generally minerotrophic (waters contain dissolved materials from soils) with the exception of ombrotrophic bogs that are fed only by water from precipitation.
Because bogs receive most of their water from the atmosphere, their water usually has low mineral ionic composition. In contrast, wetlands fed by groundwater or tides have a higher concentration of dissolved nutrients and minerals.
Fen peatlands receive water both from precipitation and ground water in varying amounts so their water chemistry ranges from acidic with low levels of dissolved minerals to alkaline with high accumulation of calcium and magnesium.[31]
Role of salinity
Salinity has a strong influence on wetland water chemistry, particularly in coastal wetlands[1][32] and in arid and semiarid regions with large precipitation deficits. Natural salinity is regulated by interactions between ground and surface water, which may be influenced by human activity.[33]
Soil
Carbon is the major nutrient cycled within wetlands. Most nutrients, such as sulfur, phosphorus, carbon, and nitrogen are found within the soil of wetlands. Anaerobic and aerobic respiration in the soil influences the nutrient cycling of carbon, hydrogen, oxygen, and nitrogen,[34] and the solubility of phosphorus[35] thus contributing to the chemical variations in its water. Wetlands with low pH and saline conductivity may reflect the presence of acid sulfates[36] and wetlands with average salinity levels can be heavily influenced by calcium or magnesium. Biogeochemical processes in wetlands are determined by soils with low redox potential.[37] Wetland soils are identified by redoxymorphic mottles (often from iron oxide rust) or low chroma intensity, as determined by the Munsell Color System.
Water chemistry
Due to the low dissolved oxygen (DO) content, and relatively low nutrient balance of wetland environments, most wetlands are very susceptible to alterations in water chemistry. Key factors that are assessed to determine water quality include:
- Major anion analysis: (HCO3−,Cl−,NO3−,SO42-)
- Major cation analysis (Ca2+, Mg2+, Na+, K+)
- pH
- Conductivity- conductivity increases with more dissolved ions in the water
- Turbidity
- Dissolved oxygen
- Temperature
- Total dissolved solids
- Gas emissions (carbon dioxide and methane; CO2 and CH4)
These chemical factors can be used to quantify wetland disturbances, and often provide information as to whether a wetland is fed by precipitation, surface water or groundwater, due to the different ion characteristics of the different water sources.[38] Wetlands are adept at impacting the water chemistry of streams or water bodies that interact with them, and can process ions that result from water pollution such as acid mine drainage or urban runoff.,[39][40]
Biota
The biota of a wetland system includes its plants (flora) and animals (fauna) and microbes (bacteria, fungi). The most important factor affecting the biota is the hydroperiod, or the duration of flooding.[1] Other important factors include fertility and salinity of the water or soils. The chemistry of water flowing into wetlands depends on the source of water, the geological material that it flows through[41] and the nutrients discharged from organic matter in the soils and plants at higher elevations.[42] Biota may vary within a wetland seasonally or in response to flood regimes.

Flora

There are four main groups of hydrophytes that are found in wetland systems throughout the world.[43]
Submerged wetland vegetation can grow in saline and fresh-water conditions. Some species have underwater flowers, while others have long stems to allow the flowers to reach the surface.[44] Submerged species provide a food source for native fauna, habitat for invertebrates, and also possess filtration capabilities. Examples include seagrasses and eelgrass.
Floating water plants or floating vegetation are usually small, like those in the Lemnoideae subfamily (duckweeds). Emergent vegetation like the cattails (Typha spp.), sedges (Carex spp.) and arrow arum (Peltandra virginica) rise above the surface of the water.
When trees and shrubs comprise much of the plant cover in saturated soils, those areas in most cases are called swamps.[1] The upland boundary of swamps is determined partly by water levels. This can be affected by dams[45] Some swamps can be dominated by a single species, such as silver maple swamps around the Great Lakes.[46] Others, like those of the Amazon basin, have large numbers of different tree species.[47] Other examples include cypress (Taxodium) and mangrove swamps.
Fauna
.jpg.webp)

Many species of fish are highly dependent on wetland ecosystems.[48][49] Seventy-five percent of the United States' commercial fish and shellfish stocks depend solely on estuaries to survive.[50] Tropical fish species need mangroves for critical hatchery and nursery grounds and the coral reef system for food.
Amphibians such as frogs and salamanders need both terrestrial and aquatic habitats in which to reproduce and feed. Because amphibians often inhabit depressional wetlands like prairie potholes and Carolina bays, the connectivity among these isolated wetlands is an important control of regional populations.[51] While tadpoles feed on algae, adult frogs forage on insects. Frogs are sometimes used as an indicator of ecosystem health because their thin skin permits absorption of nutrients and toxins from the surrounding environment resulting in increased extinction rates in unfavorable and polluted environmental conditions.[52]
Reptiles such as snakes, lizards, turtles, alligators and crocodiles are common in wetlands of some regions. In freshwater wetlands of the Southeastern US, alligators are common and a freshwater species of crocodile occurs in South Florida. The Florida Everglades is the only place in the world where both crocodiles and alligators coexist.[53] The saltwater crocodile inhabits estuaries and mangroves and can be seen along the Eastern coastline of Australia.[54] Snapping turtles are one of the many kinds of turtles found in wetlands.[55]
Birds, particularly waterfowl and wading birds, use wetlands extensively.[56]
Mammals of wetlands[57] include numerous small and medium-sized species such as voles, bats,[58] muskrats[59] and platypus in addition to large herbivorous and apex predator species such as the beaver,[60] coypu, swamp rabbit, Florida panther,[61] and moose. Wetlands attract many mammals due to abundant seeds, berries, and other vegetation as food for herbivores, as well as abundant populations of invertebrates, small reptiles and amphibians as prey for predators.[62]
Invertebrates of wetlands include aquatic insects (such as dragonflies, aquatic bugs and beetles, midges, mosquitoes), crustaceans (such as crabs, crayfish, shrimps, microcrustaceans), mollusks (such as clams, mussels, snails), and worms (such as polychaetes, oligochaetes, leeches), among others. Invertebrates comprise more than half of the known animal species in wetlands, and are considered the primary food web link between plants and higher animals (such as fish and birds).[63] The low oxygen conditions in wetland water and their frequent flooding and drying (daily in tidal wetlands, seasonally in temporary ponds and floodplains) prevent many invertebrates from inhabiting wetlands, and thus the invertebrate fauna of wetlands is often less diverse than some other kinds of habitat (such as streams, coral reefs, and forests). Some wetland invertebrates thrive in habitats that lack predatory fish. Many insects only inhabit wetlands as aquatic immatures (nymphs, larvae) and the flying adults inhabit upland habitats, returning to the wetlands to lay eggs. For instance, a common hoverfly Syritta pipiens inhabits wetlands as larvae (maggots), living in wet, rotting organic matter; these insects then visit terrestrial flowers as adult flies.
Algae
Algae are diverse plant-like organisms that can vary in size, color, and shape. Algae occur naturally in habitats such as inland lakes, inter-tidal zones, and damp soil and provide a food source for many animals, including some invertebrates, fish, turtles, and frogs. There are several groups of algae:
- Phytoplankton are microscopic, free-floating algae. These algae are so tiny that on average, 50 of these lined up end-to-end would only measure one millimeter. Phytoplankton are the basis of the food web in many water bodies being responsible for much of the primary production using photosynthesis to fix carbon.Filamentous algae are long strands of algal cells that can form floating mats. Periphyton (or epiphyton) are algae that grow as surface biofilms on plants, wood, and other substrates.[64]
- Chara and Nitella algae are upright algae that look like a submerged plants with roots.[65]
Greenhouse gas emissions
Depending on their characteristics, some wetlands are a significant source of methane emissions[66] and some are also emitters of nitrous oxide.[11][12] Nitrous oxide is a greenhouse gas with a global warming potential 300 times that of carbon dioxide and is the dominant ozone-depleting substance emitted in the 21st century.[67] Excess nutrients mainly from anthropogenic sources have been shown to significantly increase the N2O fluxes from wetland soils through denitrification and nitrification processes (see table below).[68][11][69] A study in the intertidal region of a New England salt marsh showed that excess levels of nutrients might increase N2O emissions rather than sequester them.[68]
| Wetland type | Location | N2O fluxa (µmol N2O m−2 h−1) | |
|---|---|---|---|
| Mangrove | Shenzhen and Hong Kong | 0.14 to 23.83 | [71] |
| Mangrove | Muthupet, South India | 0.41 to 0.77 | [72] |
| Mangrove | Bhitarkanika, East India | 0.20 to 4.73 | [73] |
| Mangrove | Pichavaram, South India | 0.89 to 1.89 | [73] |
| Mangrove | Queensland, Australia | −0.045 to 0.32 | [74] |
| Mangrove | South East Queensland, Australia | 0.091 to 1.48 | [75] |
| Mangrove | Southwest coast, Puerto Rico | 0.12 to 7.8 | [76] |
| Mangrove | Isla Magueyes, Puerto Rico | 0.05 to 1.4 | [76] |
| Salt marsh | Chesapeake Bay, US | 0.005 to 0.12 | [77] |
| Salt marsh | Maryland, US | 0.1 | [78] |
| Salt marsh | North East China | 0.1 to 0.16 | [79] |
| Salt marsh | Biebrza, Poland | −0.07 to 0.06 | [80] |
| Salt marsh | Netherlands | 0.82 to 1.64 | [81] |
| Salt marsh | Baltic Sea | −0.13 | [82] |
| Salt marsh | Massachusetts, US | −2.14 to 1.27 | [83] |
aThe flux rates are shown as hourly rates per unit area. A positive flux implies flux from soil into air; a negative flux implies flux from air into the soil.[84] Negative N2O fluxes are common and are caused by consumption by the soil.[85]
Data on nitrous oxide fluxes from wetlands in the southern hemisphere are lacking, as are ecosystem-based studies including the role of dominant organisms that alter sediment biogeochemistry. Aquatic invertebrates produce ecologically-relevant nitrous oxide emissions due to ingestion of denitrifying bacteria that live within the subtidal sediment and water column[86] and thus may also be influencing nitrous oxide production within some wetlands.
Emissions from Peat swamps in Southeast Asia
In Southeast Asia, peatswamp forests and soils are being drained, burnt, mined, and overgrazed, contributing to climate change.[87] As a result of peat drainage, the organic carbon that had built up over thousands of years and is normally under water is suddenly exposed to the air. The peat decomposes and is converted into carbon dioxide (CO2), which is then released into the atmosphere. Peat fires cause the same process to occur rapidly and in addition create enormous clouds of smoke that cross international borders, which now happens almost yearly in Southeast Asia. While peatlands constitute only 3% of the world's land area, their degradation produces 7% of all CO2 emissions.
Through the building of dams, Wetlands International is reducing the drainage of peatlands in Southeast Asia, hoping to mitigate CO2 emissions. Concurrent wetland restoration techniques include reforestation with native tree species as well as the formation of community fire brigades. This sustainable approach is now being applied in central Kalimantan and Sumatra, Indonesia.
Disturbances and human impacts
Wetlands, the functions and services they provide as well as their flora and fauna, can be affected by several types of disturbances.[88] The disturbances (sometimes termed stressors or alterations) can be human-associated or natural, direct or indirect, reversible or not, and isolated or cumulative. Disturbances exceed the levels or patterns normally found within wetlands of a particular class in a particular region. Predominant disturbances of wetlands include:[89][90]
- Enrichment/eutrophication
- Organic loading and reduced dissolved oxygen
- Contaminant toxicity
- Acidification
- Salinization
- Sedimentation
- Altered solar input (turbidity/shade)
- Vegetation removal
- Thermal alteration
- Drying/aridification
- Inundation/flooding
- Habitat fragmentation
- Other human impacts
Disturbances can be further categorized as follows:
- Minor disturbance: Stress that maintains ecosystem integrity.[91]
- Moderate disturbance: Ecosystem integrity is damaged but can recover in time without assistance.[91]
- Impairment or severe disturbance: Human intervention may be needed in order for ecosystem to recover.[91]
Just a few of the many sources of these disturbances include:[87]
- Drainage
- Development
- Over-grazing
- Mining
- Unsustainable water use
- Nutrient pollution (anthropogenic nitrogen inputs to aquatic systems have drastically effected the dissolved nitrogen content of wetlands, introducing higher nutrient availability which leads to eutrophication.[92])
- Water pollution
They can be manifested partly as:
- Water scarcity
- Impacts to endangered species
- Disruption of wildlife breeding grounds
- Imbalance in sediment load and nutrient filtration
Biodiversity loss occurs in wetland systems through land use changes, habitat destruction, pollution, exploitation of resources, and invasive species. Vulnerable, threatened, and endangered species include 17% of waterfowl, 38% of fresh-water dependent mammals, 33% of freshwater fish, 26% of freshwater amphibians, 72% of freshwater turtles, 86% of marine turtles, 43% of crocodilians and 27% of coral reef-building species. Introduced aquatic plants in different wetland systems can have large impacts. The introduction of water hyacinth, a native plant of South America into Lake Victoria in East Africa as well as duckweed into non-native areas of Queensland, Australia, have overtaken entire wetland systems overwhelming the habitats and reducing the diversity of native plants and animals. This is largely due to the phenomenal growth rates of the plants and their ability to float and grow across the entire surface of the water.
Conversion to dry land
Due to their productivity, wetlands are often converted into dry land with dykes and drains and used for agricultural purposes. The construction of dykes, and dams, has negative consequences for individual wetlands and entire watersheds.[1]: 497 Their proximity to lakes and rivers means that they are often developed for human settlement.[93] Once settlements are constructed and protected by dykes, the settlements then become vulnerable to land subsidence and ever increasing risk of flooding.[1]: 497 The Mississippi River Delta around New Orleans, Louisiana is a well-known example;[94] the Danube Delta in Europe is another.[95]
Services that wetlands provide to humans
Depending on a wetland's geographic and topographic location,[96] the functions it performs can support multiple ecosystem services, values, or benefits. United Nations Millennium Ecosystem Assessment and Ramsar Convention described wetlands as a whole to be of biosphere significance and societal importance in the following areas:[97]
- Water storage (flood control)
- Groundwater replenishment
- Shoreline stabilization and storm protection
- Water purification
- Wastewater treatment (in constructed wetlands)
- Reservoirs of biodiversity
- Pollination
- Wetland products
- Cultural values
- Recreation and tourism
- Climate change mitigation and adaptation
According to the Ramsar Convention:
The economic worth of the ecosystem services provided to society by intact, naturally functioning wetlands is frequently much greater than the perceived benefits of converting them to 'more valuable' intensive land use – particularly as the profits from unsustainable use often go to relatively few individuals or corporations, rather than being shared by society as a whole.
Unless otherwise cited, ecosystem services information is based on the following series of references.[50]
To replace these wetland ecosystem services, enormous amounts of money would need to be spent on water purification plants, dams, levees, and other hard infrastructure, and many of the services are impossible to replace.
Service as storage reservoirs and flood protection
Major wetland type: floodplain and closed-depression wetlands
The wetland system of floodplains is formed from major rivers downstream from their headwaters. "The floodplains of major rivers act as natural storage reservoirs, enabling excess water to spread out over a wide area, which reduces its depth and speed. Wetlands close to the headwaters of streams and rivers can slow down rainwater runoff and spring snowmelt so that it doesn't run straight off the land into water courses. This can help prevent sudden, damaging floods downstream."[50] Notable river systems that produce wide floodplains include the Nile River, the Niger river inland delta, the Zambezi River flood plain, the Okavango River inland delta, the Kafue River flood plain, the Lake Bangweulu flood plain (Africa), Mississippi River (USA), Amazon River (South America), Yangtze River (China), Danube River (Central Europe) and Murray-Darling River (Australia).
Drainage of floodplains or development activities that narrow floodplain corridors (such as the construction of levees) reduces the ability of coupled river-floodplain systems to control flood damage. That is because modified and less expansive systems must still manage the same amount of precipitation, causing flood peaks to be higher or deeper and floodwaters to travel faster.
Water management engineering developments in the past century have degraded floodplain wetlands through the construction of artificial embankments such as dykes, bunds, levees, weirs, barrages and dams. All concentrate water into a main channel and waters that historically spread slowly over a large, shallow area are concentrated. Loss of wetland floodplains results in more severe and damaging flooding. Catastrophic human impact in the Mississippi River floodplains was seen in death of several hundred individuals during a levee breach in New Orleans caused by Hurricane Katrina. Human-made embankments along the Yangtze River floodplains have caused the main channel of the river to become prone to more frequent and damaging flooding.[98] Some of these events include the loss of riparian vegetation, a 30% loss of the vegetation cover throughout the river's basin, a doubling of the percentage of the land affected by soil erosion, and a reduction in reservoir capacity through siltation build-up in floodplain lakes.[50]
Service in groundwater replenishment
Major wetland type: marsh, swamp, and subterranean karst and cave hydrological systems
The surface water which is the water visibly seen in wetland systems only represents a portion of the overall water cycle which also includes atmospheric water and groundwater. Wetland systems are directly linked to groundwater and a crucial regulator of both the quantity and quality of water found below the ground. Wetland systems that are made of permeable sediments like limestone or occur in areas with highly variable and fluctuating water tables especially have a role in groundwater replenishment or water recharge. Sediments that are porous allow water to filter down through the soil and overlying rock into aquifers which are the source of 95% of the world's drinking water. Wetlands can also act as recharge areas when the surrounding water table is low and as a discharge zone when it is too high. Karst (cave) systems are a unique example of this system and are a connection of underground rivers influenced by rain and other forms of precipitation. These wetland systems are capable of regulating changes in the water table on upwards of 130 m (430 ft).
Shoreline stabilization and storm protection
Wetland type: Mangroves, coral reefs, salt marsh
Tidal and inter-tidal wetland systems protect and stabilize coastal zones. Coral reefs provide a protective barrier to coastal shoreline. Mangroves stabilize the coastal zone from the interior and will migrate with the shoreline to remain adjacent to the boundary of the water. The main conservation benefit these systems have against storms and storm surges is the ability to reduce the speed and height of waves and floodwaters.
The number of people who live and work near the coast is expected to grow immensely over the next fifty years. From an estimated 200 million people that currently live in low-lying coastal regions, the development of urban coastal centers is projected to increase the population by fivefold within 50 years.[99] The United Kingdom has begun the concept of managed coastal realignment. This management technique provides shoreline protection through restoration of natural wetlands rather than through applied engineering. In East Asia, reclamation of coastal wetlands has resulted in widespread transformation of the coastal zone, and up to 65% of coastal wetlands have been destroyed by coastal development.[100][101] One analysis using the impact of hurricanes versus storm protection provided naturally by wetlands projected the value of this service at US$33,000/hectare/year.[102]
Water purification
Wetland types: floodplain, closed-depression wetlands, mudflat, freshwater marsh, salt marsh, mangroves
Nutrient retention: Wetlands cycle both sediments and nutrients balancing terrestrial and aquatic ecosystems. A natural function of wetland vegetation is the up-take, storage, and (for nitrate) the removal of nutrients found in runoff from the surrounding soil and water.[103] In many wetlands, nutrients are retained until plants die or are harvested by animals or humans and taken to another location, or until microbial processes convert soluble nutrients to a gas as is the case with nitrate.
Sediment and heavy metal traps: Precipitation and surface runoff induces soil erosion, transporting sediment in suspension into and through waterways. These sediments move towards larger and more sizable waterways through a natural process that moves water towards oceans. All types of sediments which may be composed of clay, sand, silt, and rock can be carried into wetland systems through this process. Wetland vegetation acts as a physical barrier to slow water flow and trap sediment for short or long periods of time. Suspended sediment often contains heavy metals that are retained when wetlands trap the sediment. In some cases, certain metals are taken up through wetland plant stems, roots, and leaves. Many floating plant species, for example, can absorb and filter heavy metals. Water hyacinth (Eichhornia crassipes), duckweed (Lemna) and water fern (Azolla) store iron and copper commonly found in wastewater, these plants also reduce pathogens. Many fast-growing plants rooted in the soils of wetlands such as cattail (Typha) and reed (Phragmites) also aid in the role of heavy metal up-take. Animals such as the oyster can filter more than 200 litres (53 US gal) of water per day while grazing for food, removing nutrients, suspended sediments, and chemical contaminants in the process. On the other hand, some types of wetlands facilitate the mobilization and bioavailability of mercury (another heavy metal), which in its methyl mercury form increases the risk of bioaccumulation in fish important to animal food webs and harvested for human consumption.
Capacity: The ability of wetland systems to store or remove nutrients and trap sediment and associated metals is highly efficient and effective but each system has a threshold. An overabundance of nutrient input from fertilizer run-off, sewage effluent, or non-point pollution will cause eutrophication. Upstream erosion from deforestation can overwhelm wetlands making them shrink in size and cause dramatic biodiversity loss through excessive sedimentation load. Retaining high levels of metals in sediments is problematic if the sediments become resuspended or oxygen and pH levels change at a future time. The capacity of wetland vegetation to store heavy metals depends on the particular metal, oxygen and pH status of wetland sediments and overlying water, water flow rate (detention time), wetland size, season, climate, type of plant, and other factors.
The capacity of a wetland to store sediment, nutrients, and metals can be diminished if sediments are compacted such as by vehicles or heavy equipment, or are regularly tilled. Unnatural changes in water levels and water sources also can affect the water purification function. If water purification functions are impaired, excessive loads of nutrients enter waterways and cause eutrophication. This is of particular concern in temperate coastal systems.[104][105] The main sources of coastal eutrophication are industrially made nitrogen, which is used as fertilizer in agricultural practices, as well as septic waste runoff.[106] Nitrogen is the limiting nutrient for photosynthetic processes in saline systems, however in excess, it can lead to an overproduction of organic matter that then leads to hypoxic and anoxic zones within the water column.[107] Without oxygen, other organisms cannot survive, including economically important finfish and shellfish species.
Wastewater treatment
An example of how a natural wetland is used to provide some degree of sewage treatment is the East Kolkata Wetlands in Kolkata, India. The wetlands cover 125 square kilometres (48 sq mi), and are used to treat Kolkata's sewage. The nutrients contained in the wastewater sustain fish farms and agriculture.

A constructed wetland (CW) is an artificial wetland to treat sewage, greywater, stormwater runoff or industrial wastewater. It may also be designed for land reclamation after mining, or as a mitigation step for natural areas lost to land development. Constructed wetlands are engineered systems that use the natural functions of vegetation, soil, and organisms to provide secondary treatment to wastewater. The design of the constructed wetland has to be adjusted according to the type of wastewater to be treated. Constructed wetlands have been used in both centralized and decentralized wastewater systems. Primary treatment is recommended when there is a large amount of suspended solids or soluble organic matter (measured as BOD and COD).[108]
Similar to natural wetlands, constructed wetlands also act as a biofilter and/or can remove a range of pollutants (such as organic matter, nutrients, pathogens, heavy metals) from the water. Constructed wetlands are designed to remove water pollutants such as suspended solids, organic matter and nutrients (nitrogen and phosphorus).[108] All types of pathogens (i.e., bacteria, viruses, protozoans and helminths) are expected to be removed to some extent in a constructed wetland. Subsurface wetlands provide greater pathogen removal than surface wetlands.[108]
There are two main types of constructed wetlands: subsurface flow and surface flow constructed wetlands. The planted vegetation plays an important role in contaminant removal. The filter bed, consisting usually of sand and gravel, has an equally important role to play.[109] Some constructed wetlands may also serve as a habitat for native and migratory wildlife, although that is not their main purpose. Subsurface flow constructed wetlands are designed to have either horizontal flow or vertical flow of water through the gravel and sand bed. Vertical flow systems have a smaller space requirement than horizontal flow systems.Reservoirs of biodiversity
Wetland systems' rich biodiversity is becoming a focal point at International Treaty Conventions and within the World Wildlife Fund organization due to the high number of species present in wetlands, the small global geographic area of wetlands, the number of species which are endemic to wetlands, and the high productivity of wetland systems. Hundred of thousands of animal species, 20,000 of them vertebrates, are living in wetland systems. The discovery rate of fresh water fish is at 200 new species per year. The impact of maintaining biodiversity is seen at the local level through job creation, sustainability, and community productivity. A good example is the Lower Mekong basin which runs through Cambodia, Laos, and Vietnam. Supporting over 55 million people, the sustainability of the region is enhanced through wildlife tours. The U.S. state of Florida has estimated that US$1.6 billion was generated in state revenue from recreational activities associated with wildlife.
Biodiverse river basins: The Amazon holds 3,000 species of freshwater fish species within the boundaries of its basin, whose function it is to disperse the seeds of trees. One of its key species, the Piramutaba catfish, Brachyplatystoma vaillantii, migrates more than 3,300 km (2,100 mi) from its nursery grounds near the mouth of the Amazon River to its spawning grounds in Andean tributaries, 400 m (1,300 ft) above sea level, distributing plants seed along the route.
Productive intertidal zones: Intertidal mudflats have a level of productivity similar to that of some wetlands even while possessing a low number of species. The abundance of invertebrates found within the mud are a food source for migratory waterfowl.
Critical life-stage habitat: Mudflats, saltmarshes, mangroves, and seagrass beds have high levels of both species richness and productivity, and are home to important nursery areas for many commercial fish stocks.
Genetic diversity: Populations of many species are confined geographically to only one or a few wetland systems, often due to the long period of time that the wetlands have been physically isolated from other aquatic sources. For example, the number of endemic species in Lake Baikal in Russia classifies it as a hotspot for biodiversity and one of the most biodiverse wetlands in the entire world. Evidence from a research study by Mazepova et al. suggest that the number of crustacean species endemic to Baikal Lake (over 690 species and subspecies) exceeds the number of the same groups of animals inhabiting all the fresh water bodies of Eurasia together. Its 150 species of free-living Platyhelminthes alone is analogous to the entire number in all of Eastern Siberia. The 34 species and subspecies number of Baikal sculpins is more than twice the number of the analogous fauna that inhabits Eurasia. In southern Baikal, about 300 species of free-living nematodes were found in only six near-shore sampling localities. "If we will take into consideration, that about 60% of the animals can be found nowhere else except Baikal, it may be assumed that the lake may be the biodiversity center of the Eurasian continent."[110]
Wetland products and productivity

Wetland productivity is linked to the climate, wetland type, and nutrient availability. Low water and occasional drying of the wetland bottom during droughts (dry marsh phase) stimulate plant recruitment from a diverse seed bank and increase productivity by mobilizing nutrients. In contrast, high water during deluges (lake marsh phase) causes turnover in plant populations and creates greater interspersion of element cover and open water, but lowers overall productivity. During a cover cycle that ranges from open water to complete vegetation cover, annual net primary productivity may vary 20-fold.[111] The grasses of fertile floodplains such as the Nile produce the highest yield including plants such as Arundo donax (giant reed), Cyperus papyrus (papyrus), Phragmites (reed) and Typha,
Wetlands naturally produce an array of vegetation and other ecological products that can be harvested for personal and commercial use.[112] The most significant of these is fish which have all or part of their life-cycle occur within a wetland system. Fresh and saltwater fish are the main source of protein for one billion people and comprise 15% of an additional two billion people's diets. In addition, fish generate a fishing industry that provides 80% of the income and employment to residents in developing countries. Another food staple found in wetland systems is rice, a popular grain that is consumed at the rate of one fifth of the total global calorie count. In Bangladesh, Cambodia and Vietnam, where rice paddies are predominant on the landscape, rice consumption reach 70%.[113] Some native wetland plants in the Caribbean and Australia are harvested sustainably for medicinal compounds; these include the red mangrove (Rhizophora mangle) which possesses antibacterial, wound-healing, anti-ulcer effects, and antioxidant properties.[113]
Food converted to sweeteners and carbohydrates include the sago palm of Asia and Africa (cooking oil), the nipa palm of Asia (sugar, vinegar, alcohol, and fodder) and honey collection from mangroves. More than supplemental dietary intake, this produce sustains entire villages. Coastal Thailand villages earn the key portion of their income from sugar production while the country of Cuba relocates more than 30,000 hives each year to track the seasonal flowering of the mangrove Avicennia.
Other mangrove-derived products:
- Fuelwood
- Salt (produced by evaporating seawater)
- Animal fodder
- Traditional medicines (e.g. from mangrove bark)
- Fibers for textiles
- Dyes and tannins
Over-fishing is the major problem for sustainable use of wetlands. Concerns are developing over certain aspects of farm fishing, which uses natural waterways to harvest fish for human consumption and pharmaceuticals. This practice has become especially popular in Asia and the South Pacific. Its impact upon much larger waterways downstream has negatively affected many small island developing states.[114]
Aquaculture is continuing to develop rapidly throughout the Asia-Pacific region specifically in China with world holdings in Asia equal to 90% of the total number of aquaculture farms and 80% of its global value.[113] Some aquaculture has eliminated massive areas of wetland through practices seen such as in the shrimp farming industry's destruction of mangroves. Even though the damaging impact of large scale shrimp farming on the coastal ecosystem in many Asian countries has been widely recognized for quite some time now, it has proved difficult to check in absence of other employment avenues for people engaged in such occupation. Also burgeoning demand for shrimps globally has provided a large and ready market for the produce.
Climate change mitigation and adaptation
Wetlands perform two important functions in relation to climate change. They have mitigation effects through their ability to sink carbon, converting a greenhouse gas (carbon dioxide) to solid plant material through the process of photosynthesis, and also through their ability to store and regulate water.[115][116] Wetlands store approximately 44.6 million tonnes of carbon per year globally.[117] In salt marshes and mangrove swamps in particular, the average carbon sequestration rate is 210 g CO2 m−2 y−1 while peatlands sequester approximately 20–30 g CO2 m−2 y−1.[117][118] Coastal wetlands, such as tropical mangroves and some temperate salt marshes, are known to be sinks for carbon that otherwise contributes to climate change in its gaseous forms (carbon dioxide and methane). The ability of many tidal wetlands to store carbon and minimize methane flux from tidal sediments has led to sponsorship of blue carbon initiatives that are intended to enhance those processes.[119]
Additional functions and uses of wetlands
Some types of wetlands can serve as fire breaks that help slow the spread of minor wildfires. Larger wetland systems can influence local precipitation patterns. Some boreal wetland systems in catchment headwaters may help extend the period of flow and maintain water temperature in connected downstream waters. Pollination services are supported by many wetlands which may provide the only suitable habitat for pollinating insects, birds, and mammals in highly developed areas.
Conservation

Wetlands have historically been the victim of large draining efforts for real estate development, or flooding for use as recreational lakes or hydropower generation. Some of the world's most important agricultural areas are wetlands that have been converted to farmland.[120][121][122][123] Since the 1970s, more focus has been put on preserving wetlands for their natural function yet by 1993 half the world's wetlands had been drained.[124]
In order to maintain wetlands and sustain their functions, alterations and disturbances that are outside the normal range of variation should be minimized.
Balancing wetland conservation with the needs of people
Wetlands are vital ecosystems that provide livelihoods for the millions of people who live in and around them. The Millennium Development Goals (MDGs) called for different sectors to join forces to secure wetland environments in the context of sustainable development and improving human wellbeing. Studies have shown that it is possible to conserve wetlands while improving the livelihoods of people living among them. Case studies conducted in Malawi and Zambia looked at how dambos – wet, grassy valleys or depressions where water seeps to the surface – can be farmed sustainably to improve livelihoods. Project outcomes included a high yield of crops, development of sustainable farming techniques, and adequate water management generating enough water for use as irrigation.[125]
Ramsar Convention
The Convention on Wetlands of International Importance, especially as Waterfowl Habitat, or Ramsar Convention, is an international treaty designed to address global concerns regarding wetland loss and degradation. The primary purposes of the treaty are to list wetlands of international importance and to promote their wise use, with the ultimate goal of preserving the world's wetlands. Methods include restricting access to the majority portion of wetland areas, as well as educating the public to combat the misconception that wetlands are wastelands. The Convention works closely with five International Organisation Partners. These are: Birdlife International, the IUCN, the International Water Management Institute, Wetlands International and the World Wide Fund for Nature. The partners provide technical expertise, help conduct or facilitate field studies and provide financial support. The IOPs also participate regularly as observers in all meetings of the Conference of the Parties and the Standing Committee and as full members of the Scientific and Technical Review Panel.
Valuation
The value of a wetland to local communities typically involves first mapping a region's wetlands, then assessing the functions and ecosystem services the wetlands provide individually and cumulatively, and evaluating that information to prioritize or rank individual wetlands or wetland types for conservation, management, restoration, or development. Over a longer period, it requires keeping inventories of known wetlands and monitoring a representative sample of the wetlands to determine changes due to both natural and human factors.
Assessment
Rapid assessment methods are used to score, rank, rate, or categorize various functions, ecosystem services, species, communities, levels of disturbance, and/or ecological health of a wetland or group of wetlands. This is often done to prioritize particular wetlands for conservation (avoidance) or to determine the degree to which loss or alteration of wetland functions should be compensated, such as by restoring degraded wetlands elsewhere or providing additional protections to existing wetlands. Rapid assessment methods are also applied before and after a wetland has been restored or altered, to help monitor or predict the effects of those actions on various wetland functions and the services they provide. Assessments are typically considered to be "rapid" when they require only a single visit to the wetland lasting less than one day, which in some cases may include interpretation of aerial imagery and geographic information system (GIS) analyses of existing spatial data, but not detailed post-visit laboratory analyses of water or biological samples.
To achieve consistency among persons doing the assessment, rapid methods present indicator variables as questions or checklists on standardized data forms, and most methods standardize the scoring or rating procedure that is used to combine question responses into estimates of the levels of specified functions relative to the levels estimated in other wetlands ("calibration sites") assessed previously in a region.[126] Rapid assessment methods, partly because they often use dozens of indicators pertaining to conditions surrounding a wetland as well as within the wetland itself, aim to provide estimates of wetland functions and services that are more accurate and repeatable than simply describing a wetland's class type.[3] A need for wetland assessments to be rapid arises mostly when government agencies set deadlines for decisions affecting a wetland, or when the number of wetlands needing information on their functions or condition is large.
Inventory
Although developing a global inventory of wetlands has proven to be a large and difficult undertaking, many efforts at more local scales have been successful. Current efforts are based on available data, but both classification and spatial resolution have sometimes proven to be inadequate for regional or site-specific environmental management decision-making. It is difficult to identify small, long, and narrow wetlands within the landscape. Many of today's remote sensing satellites do not have sufficient spatial and spectral resolution to monitor wetland conditions, although multispectral IKONOS and QuickBird data may offer improved spatial resolutions once it is 4 m or higher. Majority of the pixels are just mixtures of several plant species or vegetation types and are difficult to isolate which translates into an inability to classify the vegetation that defines the wetland.
Monitoring and mapping
A wetland needs to be monitored over time to assess whether it is functioning at an ecologically sustainable level or whether it is becoming degraded. Degraded wetlands will suffer a loss in water quality, loss of sensitive species, and aberrant functioning of soil geochemical processes.
Practically, many natural wetlands are difficult to monitor from the ground as they quite often are difficult to access and may require exposure to dangerous plants and animals as well as diseases borne by insects or other invertebrates. Therefore, mapping using aerial imagery is one effective tool to monitor a wetland, especially a large wetland, and can also be used to monitor the status of numerous wetlands throughout a watershed or region. Many remote sensing methods can be used to map wetlands. Remote-sensing technology permits the acquisition of timely digital data on a repetitive basis. This repeat coverage allows wetlands, as well as the adjacent land-cover and land-use types, to be monitored seasonally and/or annually. Using digital data provides a standardized data-collection procedure and an opportunity for data integration within a geographic information system.
Restoration
Restoration and restoration ecologists intend to return wetlands to their natural trajectory by aiding directly with the natural processes of the ecosystem.[91] These direct methods vary with respect to the degree of physical manipulation of the natural environment and each are associated with different levels of restoration.[91] Restoration is needed after disturbance or perturbation of a wetland.[91] Disturbances include exogenous factors such as flooding or drought.[91] Other external damage may be anthropogenic disturbance caused by clear-cut harvesting of trees, oil and gas extraction, poorly defined infrastructure installation, over grazing of livestock, ill-considered recreational activities, alteration of wetlands including dredging, draining, and filling, and other negative human impacts.[91][18] Disturbance puts different levels of stress on an environment depending on the type and duration of disturbance.[91] There is no one way to restore a wetland and the level of restoration required will be based on the level of disturbance although, each method of restoration does require preparation and administration.[91]
Levels of restoration
Factors influencing selected approach may include[91]
- Budget
- Time scale limitations
- Project goals
- Level of disturbance
- Landscape and ecological constraints
- Political and administrative agendas
- Socioeconomic priorities
Prescribed natural regeneration
There are no biophysical manipulation and the ecosystem is left to recover based on the process of succession alone.[91] The focus of this method is to eliminate and prevent further disturbance from occurring.[91] In order for this type of restoration to be effective and successful there must be prior research done to understand the probability that the wetland will recover with this method.[91] Otherwise, some biophysical manipulation may be required to enhance the rate of succession to an acceptable level determined by the project managers and ecologists.[91] This is likely to be the first method of approach for the lowest level of disturbance being that it is the least intrusive and least costly.[91]
Assisted natural regeneration
There are some biophysical manipulations however they are non-intrusive.[91] Example methods that are not limited to wetlands include prescribed burns to small areas, promotion of site specific soil microbiota and plant growth using nucleation planting whereby plants radiate from an initial planting site,[127] and promotion of niche diversity or increasing the range of niches to promote use by a variety of different species.[91] These methods can make it easier for the natural species to flourish by removing competition from their environment and can speed up the process of succession.[91]
Partial reconstruction
Here there is a mix between natural regeneration and manipulated environmental control.[91] These manipulations may require some engineering and more invasive biophysical manipulation including ripping of subsoil, agrichemical applications such as herbicides and insecticides, laying of mulch, mechanical seed dispersal, and tree planting on a large scale.[91] In these circumstances the wetland is impaired and without human assistance it would not recover within an acceptable period of time determined by ecologists.[91] Again these methods of restoration will have to be considered on a site by site basis as each site will require a different approach based on levels of disturbance and ecosystem dynamics.[91]
Complete reconstruction
The most expensive and intrusive method of reconstruction requiring engineering and ground up reconstruction.[91] Because there is a redesign of the entire ecosystem it is important that the natural trajectory of the ecosystem be considered and that the plant species will eventually return the ecosystem towards its natural trajectory.[91]
Legislation
International Efforts
- Ramsar Convention[18]
- North American Waterfowl Management Plan[18]
United States
Each county and region tends to have its own definition for legal purposes. In the United States, wetlands are defined as "those areas that are inundated or saturated by surface or groundwater at a frequency and duration sufficient to support, and that under normal circumstances do support, a prevalence of vegetation typically adapted for life in saturated soil conditions. Wetlands generally include swamps, marshes, bogs and similar areas".[128] This definition has been used in the enforcement of the Clean Water Act. Some US states, such as Massachusetts and New York, have separate definitions that may differ from the federal government's.
In the United States Code, the term wetland is defined "as land that (A) has a predominance of hydric soils, (B) is inundated or saturated by surface or groundwater at a frequency and duration sufficient to support a prevalence of hydrophytic vegetation typically adapted for life in saturated soil conditions and (C) under normal circumstances supports a prevalence of such vegetation." Related to this legal definitions, the term "normal circumstances" are conditions expected to occur during the wet portion of the growing season under normal climatic conditions (not unusually dry or unusually wet), and in the absence of significant disturbance. It is not uncommon for a wetland to be dry for long portions of the growing season. Wetlands can be dry during the dry season and abnormally dry periods during the wet season, but under normal environmental conditions the soils in a wetland will be saturated to the surface or inundated such that the soils become anaerobic, and those conditions will persist through the wet portion of the growing season.[129]
Largest wetlands
The largest wetlands include the swamp forests of the Amazon River basin, the peatlands of the West Siberian Plain,[6] the Pantanal in South America,[7] and the Sundarbans in the Ganges-Brahmaputra delta.[8]
See also
- A Directory of Important Wetlands in Australia
- Converted wetland
- Groundwater-dependent ecosystems
- List of Ramsar wetland sites in Pakistan
- Mediterranean wetlands
- Paludification
- Slough
- All pages with titles containing wetland
References
- Keddy, P.A. (2010). Wetland ecology : principles and conservation (2nd ed.). New York: Cambridge University Press. ISBN 978-0521519403.
- "Official page of the Ramsar Convention". Retrieved 2011-09-25.
- Dorney, J.; Savage, R.; Adamus, P.; Tiner, R., eds. (2018). Wetland and Stream Rapid Assessments: Development, Validation, and Application. London; San Diego, CA: Academic Press. ISBN 978-0-12-805091-0. OCLC 1017607532.
- Davidson, N.C. (2014). "How much wetland has the world lost? Long-term and recent trends in global wetland area". Marine and Freshwater Research. 65 (10): 934–941. doi:10.1071/MF14173. S2CID 85617334.
- "US EPA". 2015-09-18. Retrieved 2011-09-25.
- Fraser, L.; Keddy, P.A., eds. (2005). The World's Largest Wetlands: Their Ecology and Conservation. Cambridge, UK: Cambridge University Press. ISBN 978-0521834049.
- "WWF Pantanal Programme". Retrieved 2011-09-25.
- Giri, C.; Pengra, B.; Zhu, Z.; Singh, A.; Tieszen, L.L. (2007). "Monitoring mangrove forest dynamics of the Sundarbans in Bangladesh and India using multi-temporal satellite data from 1973 to 2000". Estuarine, Coastal and Shelf Science. 73 (1–2): 91–100. Bibcode:2007ECSS...73...91G. doi:10.1016/j.ecss.2006.12.019.
- "Wetlands". USDA- Natural Resource Conservation Center.
- Davidson, N.C.; D'Cruz, R. & Finlayson, C.M. (2005). Ecosystems and Human Well-being: Wetlands and Water Synthesis: a report of the Millennium Ecosystem Assessment (PDF). Washington, DC: World Resources Institute. ISBN 978-1-56973-597-8.
- Bange, Hermann W. (2006). "Nitrous oxide and methane in European coastal waters". Estuarine, Coastal and Shelf Science. 70 (3): 361–374. Bibcode:2006ECSS...70..361B. doi:10.1016/j.ecss.2006.05.042.
- Thompson, A. J.; Giannopoulos, G.; Pretty, J.; Baggs, E. M.; Richardson, D. J. (2012). "Biological sources and sinks of nitrous oxide and strategies to mitigate emissions". Philosophical Transactions of the Royal Society B. 367 (1593): 1157–1168. doi:10.1098/rstb.2011.0415. PMC 3306631. PMID 22451101.
- "Home : Oxford English Dictionary". www.oed.com. Retrieved 2022-07-08.
- US EPA, OW (2015-09-18). "What is a Wetland?". US EPA. Retrieved 2022-07-08.
- "Glossary of Terms". Carpinteria Valley Water District. Archived from the original on April 25, 2012. Retrieved 2012-05-23.
- "Glossary". Mapping2.orr.noaa.gov. Archived from the original on 2012-04-25. Retrieved 2012-05-23.
- "Glossary". Alabama Power. Archived from the original on 2012-03-21. Retrieved 2012-05-23.
- Mitsch, William J.; Gosselink, James G. (2007-08-24). Wetlands (4th ed.). New York, NY: John Wiley & Sons. ISBN 978-0-471-69967-5.
- "The Ramsar 40th Anniversary Message for November". Ramsar. Retrieved 2011-10-10.
- Environmental Laboratory. (1987). Corps of Engineers wetlands delineation manual. Tech. Rep. Y‐87–1.
- Sharitz, Rebecca R.; Batzer, Darold P.; Pennings, Steven C. (2019-12-31), "1. Ecology of Freshwater and Estuarine Wetlands: An Introduction", Ecology of Freshwater and Estuarine Wetlands, Berkeley: University of California Press, pp. 1–22, doi:10.1525/9780520959118-003, ISBN 978-0-520-95911-8, S2CID 198427881, retrieved 2022-08-12
- US EPA, OW (2015-09-18). "What is a Wetland?". www.epa.gov. Retrieved 2022-08-12.
- "Wetland Types | Department of Environmental Conservation".
- A Directory of Important Wetlands in Australia: Third edition, Chapter 2: Wetland classification system, Criteria for inclusion and Data presentation. Australian Department of the Environment. 2001. Retrieved 30 March 2021.
{{cite book}}: CS1 maint: url-status (link) - "NPWRC :: Classification of Wetlands and Deepwater Habitats of the United States". www.fws.gov. Archived from the original on 2014-01-21. Retrieved 2018-07-28.
- Watson, G. E. (2006). Big Thicket Plant Ecology: An Introduction. Temple Big Thicket Series #5 (Third ed.). Denton, Texas: University of North Texas Press. ISBN 978-1574412147.
- Texas Parks and Wildlife. Ecological Mapping systems of Texas: West Gulf Coastal Plain Seepage Swamp and Baygall. Retrieved 7 July 2020
- "PEATLANDS, CLIMATE CHANGE MITIGATION AND BIODIVERSITY CONSERVATION".
- "Ramsar Convention Technical Reports".
- Richardson, J. L.; Arndt, J. L.; Montgomery, J. A. (2001). "Hydrology of wetland and related soils". In Richardson, J. L.; Vepraskas, M. J. (eds.). Wetland Soils. Boca Raton, FL: Lewis Publishers.
- Vitt, D. H.; Chee, W (1990). "The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada". Plant Ecology. 89 (2): 87–106. doi:10.1007/bf00032163. S2CID 25071105.
- Silliman, B. R.; Grosholz, E. D.; Bertness, M. D., eds. (2009). Human Impacts on Salt Marshes: A Global Perspective. Berkeley, CA: University of California Press.
- Smith, M. J.; Schreiber, E. S. G.; Kohout, M.; Ough, K.; Lennie, R.; Turnbull, D.; Jin, C.; Clancy, T. (2007). "Wetlands as landscape units: spatial patterns in salinity and water chemistry". Wetlands, Ecology & Management. 15 (2): 95–103. doi:10.1007/s11273-006-9015-5. S2CID 20196854.
- Ponnamperuma, F. N. (1972). The chemistry of submerged soils. Advances in Agronomy. Vol. 24. pp. 29–96. doi:10.1016/S0065-2113(08)60633-1. ISBN 9780120007240.
- Moore, P. A. Jr.; Reddy, K. R. (1994). "Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida". Journal of Environmental Quality. 23 (5): 955–964. doi:10.2134/jeq1994.00472425002300050016x. PMID 34872208.
- Minh, L. Q.; Tuong, T. P.; van Mensvoort, M. E. F.; Bouma, J. (1998). "Soil and water table management effects on aluminum dynamics in an acid sulphate soil in Vietnam". Agriculture, Ecosystems & Environment. 68 (3): 255–262. doi:10.1016/s0167-8809(97)00158-8.
- Schlesinger, W. A. (1997). Biogeochemistry: An Analysis of Global Change (2nd ed.). San Diego, CA: Academic Press. ISBN 9780126251555.
- Arthington, Angela H. (2012-10-15), "Wetlands, Threats, and Water Requirements", Environmental Flows, University of California Press, pp. 243–258, doi:10.1525/california/9780520273696.003.0017, ISBN 9780520273696
- Kelman Wieder, R.; Lang, GeraldE. (November 1984). "Influence of wetlands and coal mining on stream water chemistry". Water, Air, and Soil Pollution. 23 (4): 381. Bibcode:1984WASP...23..381K. doi:10.1007/bf00284734. ISSN 0049-6979. S2CID 96209351.
- Jones, C Nathan; McLaughlin, Daniel L; Henson, Kevin; Haas, Carola A; Kaplan, David A (2018-01-10). "From salamanders to greenhouse gases: does upland management affect wetland functions?". Frontiers in Ecology and the Environment. 16 (1): 14–19. doi:10.1002/fee.1744. ISSN 1540-9295. S2CID 90980246.
- Bedford, B. L. (1996). "The need to define hydrologic equivalence at the landscape scale for freshwater wetland mitigation". Ecological Applications. 6 (1): 57–68. doi:10.2307/2269552. JSTOR 2269552.
- Nelson, M. L.; Rhoades, C. C.; Dwire, K. A. (2011). "Influences of Bedrock Geology on Water Chemistry of Slope Wetlands and Headwaters Streams in the Southern Rocky Mountains". Wetlands. 31 (2): 251–261. doi:10.1007/s13157-011-0157-8. S2CID 14521026.
- "Blacktown Council wetlands". Archived from the original on 2011-04-10. Retrieved 2011-09-25.
- Hutchinson, G. E. (1975). A Treatise on Limnology. Vol. 3: Limnological Botany. New York, NY: John Wiley.
- Hughes, F. M. R., ed. (2003). The Flooded Forest: Guidance for policy makers and river managers in Europe on the restoration of floodplain forests. FLOBAR2, Department of Geography, University of Cambridge, Cambridge, UK.
- Wilcox, D. A; Thompson, T. A.; Booth, R. K.; Nicholas, J. R. (2007). Lake-level variability and water availability in the Great Lakes. USGS Circular 1311.
- Goulding, M. (1980). The Fishes and the Forest: Explorations in Amazonian Natural History. Berkeley, CA: University of California Press.
- Colvin, Susan A. R.; Sullivan, S. Mažeika P.; Shirey, Patrick D.; Colvin, Randall W.; Winemiller, Kirk O.; Hughes, Robert M.; Fausch, Kurt D.; Infante, Dana M.; Olden, Julian D.; Bestgen, Kevin R.; Danehy, Robert J.; Eby, Lisa (2019). "Headwater Streams and Wetlands are Critical for Sustaining Fish, Fisheries, and Ecosystem Services". Fisheries. 44 (2): 73–91. doi:10.1002/fsh.10229. S2CID 92052162.
- Sievers, Michael; Brown, Christopher J.; Tulloch, Vivitskaia J. D.; Pearson, Ryan M.; Haig, Jodie A.; Turschwell, Mischa P.; Connolly, Rod M. (2019-09-01). "The Role of Vegetated Coastal Wetlands for Marine Megafauna Conservation". Trends in Ecology & Evolution. 34 (9): 807–817. doi:10.1016/j.tree.2019.04.004. hdl:10072/391960. ISSN 0169-5347. PMID 31126633. S2CID 164219103.
- "Ramsar Convention Ecosystem Services Benefit Factsheets". Retrieved 2011-09-25.
- Zamberletti, Patrizia; Zaffaroni, Marta; Accatino, Francesco; Creed, Irena F.; De Michele, Carlo (2018-09-24). "Connectivity among wetlands matters for vulnerable amphibian populations in wetlandscapes". Ecological Modelling. 384: 119–127. doi:10.1016/j.ecolmodel.2018.05.008. ISSN 0304-3800. S2CID 90384249.
- "Frogs | Bioindicators". Savethefrogs.com. 2011. Retrieved 2014-01-21.
- Mazzotti, F.J.; Best, G.R.; Brandt, L.A.; Cherkiss, M.S.; Jeffery, B.M.; Rice, K.G. (2009). "Alligators and crocodiles as indicators for restoration of Everglades ecosystems". Ecological Indicators. 9 (6): S137−S149. doi:10.1016/j.ecolind.2008.06.008.
- Messel, H. 1981. Surveys of tidal river systems in the Northern Territory of Australia and their crocodile populations (Vol. 1). Pergamon Press.
- Piczak, Morgan L.; Chow-Fraser, Patricia (2019-06-01). "Assessment of critical habitat for common snapping turtles (Chelydra serpentina) in an urbanized coastal wetland". Urban Ecosystems. 22 (3): 525–537. doi:10.1007/s11252-019-00841-1. ISSN 1573-1642. S2CID 78091420.
- Milton, W. (1999). Wetland birds: habitat resources and conservation implications. Cambridge: Cambridge University Press. ISBN 978-0511011368. OCLC 50984660.
- Batzer, Darold; Boix, Dani, eds. (2016). Invertebrates in Freshwater Wetlands. Cham: Springer International Publishing. doi:10.1007/978-3-319-24978-0. ISBN 978-3-319-24976-6. S2CID 29672842.
- Mas, Maria; Flaquer, Carles; Rebelo, Hugo; López‐Baucells, Adrià (2021). "Bats and wetlands: synthesising gaps in current knowledge and future opportunities for conservation". Mammal Review. 51 (3): 369–384. doi:10.1111/mam.12243. ISSN 0305-1838. S2CID 233974999.
- Bomske, Caleb M.; Ahlers, Adam A. (2021). "How do muskrats Ondatra zibethicus affect ecosystems? A review of evidence". Mammal Review. 51 (1): 40–50. doi:10.1111/mam.12218. ISSN 0305-1838. S2CID 224916636.
- Rosell, Frank; Bozser, Orsolya; Collen, Peter; Parker, Howard (2005). "Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems". Mammal Review. 35 (3–4): 248–276. doi:10.1111/j.1365-2907.2005.00067.x. hdl:11250/2438080. ISSN 0305-1838.
- Kerk, Madelon; Onorato, David P.; Hostetler, Jeffrey A.; Bolker, Benjamin M.; Oli, Madan K. (2019). "Dynamics, Persistence, and Genetic Management of the Endangered Florida Panther Population". Wildlife Monographs. 203 (1): 3–35. doi:10.1002/wmon.1041. ISSN 0084-0173. S2CID 199641325.
- "Mammals in Wetlands". NSW Environment, Energy and Science. Department of Planning, Industry and Environment. 2020-02-20. Retrieved 2021-10-11.
Mammals live in wetlands because they are adapted to the wet conditions and there is a plentiful supply of their preferred foods. For example: The swamp rat feeds on grasses, sedges, reeds, seeds and insects. The water rat feeds on a wide range of prey including large insects, crustaceans, mussels and fishes, and even frogs, lizards, small mammals and water birds. The platypus mainly feeds during the night on a wide variety of aquatic invertebrates, free-swimming organisms such as shrimps, swimming beetles, water bugs and tadpoles, and at times worms, freshwater pea mussels and snails. The fishing bat feeds on aquatic insects, small fish and flies close to the surface of rainforest streams or large lakes and reservoirs.
- Batzer, Darold P.; Rader, Russell Ben.; Wissinger, Scott A. (1999). Invertebrates in freshwater wetlands of North America : ecology and management. New York: Wiley. ISBN 978-0471292586. OCLC 39747651.
- Wu, Yonghong; Liu, Junzhuo; Rene, Eldon R. (2018-01-01). "Periphytic biofilms: A promising nutrient utilization regulator in wetlands". Bioresource Technology. 1st International Conference on Ecotechnologies for Controlling Non-point Source Pollution and Protecting Aquatic Ecosystem. 248 (Pt B): 44–48. doi:10.1016/j.biortech.2017.07.081. ISSN 0960-8524. PMID 28756125.
- "Taken from Blacktown Council Wetland Inventory". Blacktown Council. Archived from the original on 2012-01-22. Retrieved 2012-05-23.
- Tiwari, Shashank; Singh, Chhatarpal; Singh, Jay Shankar (2020), Upadhyay, Atul Kumar; Singh, Ranjan; Singh, D. P. (eds.), "Wetlands: A Major Natural Source Responsible for Methane Emission", Restoration of Wetland Ecosystem: A Trajectory Towards a Sustainable Environment, Singapore: Springer, pp. 59–74, doi:10.1007/978-981-13-7665-8_5, ISBN 978-981-13-7665-8, S2CID 198421761, retrieved 2022-10-09
- Ravishankara, A. R.; Daniel, John S.; Portmann, Robert W. (2009). "Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century". Science. 326 (5949): 123–125. Bibcode:2009Sci...326..123R. doi:10.1126/science.1176985. PMID 19713491. S2CID 2100618.
- Moseman-Valtierra, S.; et al. (2011). "Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O". Atmospheric Environment. 45 (26): 4390–4397. Bibcode:2011AtmEn..45.4390M. doi:10.1016/j.atmosenv.2011.05.046.
- Martin, Rose M.; Wigand, Cathleen; Elmstrom, Elizabeth; Lloret, Javier; Valiela, Ivan (20 April 2018). "Long-term nutrient addition increases respiration and nitrous oxide emissions in a New England salt marsh". Ecology and Evolution. 8 (10): 4958–4966. doi:10.1002/ece3.3955. ISSN 2045-7758. PMC 5980632. PMID 29876073.
- Moseman-Valtierra, S. (2012). "Chapter 1: Reconsidering the climatic roles of marshes: Are they sinks or sources of greenhouse gases?". In Abreu, D. C.; Borbón, S. L. (eds.). Marshes: Ecology, Management and Conservation. New York, NY: Nova Science.
- Chen, G.; Tam, N.; Ye, Y. (2010). "Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China". Science of the Total Environment. 408 (13): 2761–2767. Bibcode:2010ScTEn.408.2761C. doi:10.1016/j.scitotenv.2010.03.007. PMID 20381125.
- Krithika, K.; Purvaja, R.; Ramesh, R. (2008). "Fluxes of methane and nitrous oxide from an Indian mangrove". Current Science. 94: 218–224.
- Chauhan, R.; Ramanathan, A. L.; Adhya, T. K. (2008). "Assessment of methane and nitrous oxide flux from mangroves along Eastern coast of India". Geofluids. 8 (4): 321–332. doi:10.1111/j.1468-8123.2008.00227.x.
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. (2003). "Emission of methane and nitrous oxide by Australian mangrove ecosystems". Plant Biology. 5 (4): 423–431. doi:10.1055/s-2003-42712.
- Allen, D. E.; Dalal, R. C.; Rennenberg, L.; Meyer, R.; Reeves, S.; Schmidt, S. (2007). "Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove soils and the atmosphere". Soil Biology and Biochemistry. 39 (2): 622–631. doi:10.1016/j.soilbio.2006.09.013.
- Sotomayor, D.; Corredor, J. E.; Morell, J. M. (1994). "Methane flux from mangrove soils along the southwestern coast of Puerto Rico". Estuaries. 17 (1): 140–147. doi:10.2307/1352563. JSTOR 1352563. S2CID 86450737.
- Jordan, T. E.; Andrews, M. P.; Szuch, R. P.; Whigham, D. F.; Weller, D. E.; Jacobs, A. D. (2007). "Comparing Functional Assessments Of Wetlands To Measurements Of Soil Characteristics And Nitrogen Processing" (PDF). Wetlands (Submitted manuscript). 27 (3): 479–497. doi:10.1672/0277-5212(2007)27[479:cfaowt]2.0.co;2. S2CID 9109080.
- Weller, D. E.; Cornell, D. L.; Jordan, T. E. (1994). "Denitrification in riparian forests receiving agricultural discharges". Global Wetlands: Old World and New: 117–131.
- Yu, J.; Liu, J.; Wang, J.; Sun, W.; Patrick, W. H.; Meixner, F. X. (2007). "Nitrous Oxide Emission from Deyeuxia angustifolia Freshwater Marsh in Northeast China". Environmental Management. 40 (4): 613–622. Bibcode:2007EnMan..40..613Y. doi:10.1007/s00267-006-0349-9. PMID 17661130. S2CID 16763038.
- Roobroeck, D.; Butterbach-Bahl, K.; Brüggemann, N.; Boeckx, P. (2010). "Dinitrogen and nitrous oxide exchanges from an undrained monolith fen: Short-term responses following nitrate addition". European Journal of Soil Science. 61 (5): 662–670. doi:10.1111/j.1365-2389.2010.01269.x. S2CID 94635551.
- Hefting, M. M.; Bobbink, R.; De Caluwe, H. (2003). "Nitrous Oxide Emission and Denitrification in Chronically Nitrate-Loaded Riparian Buffer Zones". Journal of Environmental Quality. 32 (4): 1194–203. doi:10.2134/jeq2003.1194. PMID 12931872.
- Liikanen, A. (2009). "Methane and nitrous oxide fluxes in two coastal wetlands in the northeastern Gulf of Bothnia, Baltic Sea". Boreal Environment Research. 14 (3): 351–368.
- Moseman-Valtierra, S.; et al. (2011). "Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O". Atmospheric Environment. 45 (26): 4390–4397. Bibcode:2011AtmEn..45.4390M. doi:10.1016/j.atmosenv.2011.05.046.
- Agricultural & Environmental Data Archive
- Audet, Joachim; Hoffmann, Carl C.; Andersen, Peter M.; Baattrup-Pedersen, Annette; Johansen, Jan R.; Larsen, Søren E.; Kjaergaard, Charlotte; Elsgaard, Lars (2014-01-01). "Nitrous oxide fluxes in undisturbed riparian wetlands located in agricultural catchments: Emission, uptake and controlling factors". Soil Biology and Biochemistry. 68: 291–299. doi:10.1016/j.soilbio.2013.10.011. ISSN 0038-0717.
- Stief, P.; Poulsen, M.; Nielsen; et al. (2009). "Nitrous oxide emission by aquatic macrofauna". Proceedings of the National Academy of Sciences. 106 (11): 4296–4300. Bibcode:2009PNAS..106.4296S. doi:10.1073/pnas.0808228106. PMC 2651200. PMID 19255427.
- "Wetlands International works to sustain and restore wetlands for people and biodiversity". Wetlands International. Retrieved 2014-01-21.
- Swindles, Graeme T.; Morris, Paul J.; Mullan, Donal J.; Payne, Richard J.; Roland, Thomas P.; Amesbury, Matthew J.; Lamentowicz, Mariusz; Turner, T. Edward; Gallego-Sala, Angela; Sim, Thomas; Barr, Iestyn D. (2019-10-21). "Widespread drying of European peatlands in recent centuries". Nature Geoscience. 12 (11): 922–928. Bibcode:2019NatGe..12..922S. doi:10.1038/s41561-019-0462-z. ISSN 1752-0908. S2CID 202908362. Alt URL
- Office of Research & Development. "Impacts on quality of inland wetlands of the United States: A survey of indicators, techniques, and applications of community-level biomonitoring data". cfpub.epa.gov. Retrieved 2018-07-27.
- Adamus, Paul; J. Danielson, Thomas; Gonyaw, Alex (2001-03-24). Indicators for Monitoring Biological Integrity of Inland Freshwater Wetlands: A Survey of North American Technical Literature (1990-2000). 13214. doi:10.13140/rg.2.2.22371.86566.
- Clewell, AF; Aronson, J (2013). Ecological restoration (2nd ed.). Washington, DC: Island Press.
- Finlay, Jacques C.; Efi Foufoula-Georgiou; Dolph, Christine L.; Hansen, Amy T. (February 2018). "Contribution of wetlands to nitrate removal at the watershed scale". Nature Geoscience. 11 (2): 127–132. Bibcode:2018NatGe..11..127H. doi:10.1038/s41561-017-0056-6. ISSN 1752-0908. S2CID 46656300.
- Alexander, David E. (1 May 1999). Encyclopedia of Environmental Science. Springer. ISBN 0-412-74050-8.
- Keddy, P.A.; Campbell, D.; McFalls, T.; Shaffer, G.P.; Moreau, R.; Dranguet, C.; Heleniak, R. (2007). "The Wetlands of Lakes Pontchartrain and Maurepas: Past, Present and Future". Environmental Reviews. 15 (NA): 43–77. doi:10.1139/a06-008. ISSN 1181-8700.
- Gastescu, P. (1993). The Danube Delta: geographical characteristics and ecological recovery. Earth and Environmental Science, 29, 57–67.
- Adamus, P.R. and L.T. Stockwell. 1983. A Method for Wetland Functional Assessment. Vol. I. Critical Review and Evaluation Concepts. FHWA-IP-82-23. Federal Highway Admin., Washington, DC.
- Ecosystems and human well-being : wetlands and water synthesis : a report of the Millennium Ecosystem Assessment. Millennium Ecosystem Assessment. Washington, DC: World Resources Institute. 2005. ISBN 1-56973-597-2. OCLC 62172810.
{{cite book}}: CS1 maint: others (link) - Li, Luqian; Lu, XiXi; Chen, Zhongyuan (2007). "River channel change during the last 50 years in the middle Yangtze River, the Jianli reach". Geomorphology. 85 (3–4): 185–196. doi:10.1016/j.geomorph.2006.03.035.
- "United Nations Environment Programme (UNEP) – Home page". Retrieved 2011-12-11.
- MacKinnon, J.; Verkuil, Y. I.; Murray, N. J. (2012), IUCN situation analysis on East and Southeast Asian intertidal habitats, with particular reference to the Yellow Sea (including the Bohai Sea), Occasional Paper of the IUCN Species Survival Commission No. 47, Gland, Switzerland and Cambridge, UK: IUCN, p. 70, ISBN 9782831712550, archived from the original on 2014-06-24
- Murray, N. J.; Clemens, R. S.; Phinn, S. R.; Possingham, H. P.; Fuller, R. A. (2014). "Tracking the rapid loss of tidal wetlands in the Yellow Sea" (PDF). Frontiers in Ecology and the Environment. 12 (5): 267–272. doi:10.1890/130260.
- "FAO". Archived from the original on 2007-09-09. Retrieved 2011-09-25.
- "Letting Nature Do the Job". Wild.org. 2008-08-01. Archived from the original on 2013-01-13. Retrieved 2012-05-23.
- Valiela, I.; Collins, G.; Kremer, J.; Lajtha, K.; Geist, M.; Seely, B.; Brawley, J.; Sham, C. H. (1997). "Nitrogen loading from coastal watersheds to receiving estuaries: New method and application". Ecological Applications. 7 (2): 358–380. CiteSeerX 10.1.1.461.3668. doi:10.2307/2269505. JSTOR 2269505.
- Nixon, S. W. (1986). "Nutrients and the productivity of estuarine and coastal marine ecosystems". Journal of the Limnological Society of South Africa. 12 (1–2): 43–71. doi:10.1080/03779688.1986.9639398.
- Galloway, J. (2003). "The Nitrogen Cascade". BioScience. 53 (4): 341–356. doi:10.1641/0006-3568(2003)053[0341:tnc]2.0.co;2. S2CID 3356400.
- Diaz, R. J.; Rosenberg, R. (2008). "Spreading Dead Zones and Consequences for Marine Ecosystems". Science. 321 (5891): 926–929. Bibcode:2008Sci...321..926D. doi:10.1126/science.1156401. PMID 18703733. S2CID 32818786.
- Maiga, Y., von Sperling, M., Mihelcic, J. 2017. Constructed Wetlands. In: J.B. Rose and B. Jiménez-Cisneros, (eds) Global Water Pathogens Project. (C. Haas, J.R. Mihelcic and M.E. Verbyla) (eds) Part 4 Management Of Risk from Excreta and Wastewater) Michigan State University, E. Lansing, MI, UNESCO.
 Material was copied from this source, which is available under a Creative Commons Attribution-ShareAlike 3.0 Unported license.
Material was copied from this source, which is available under a Creative Commons Attribution-ShareAlike 3.0 Unported license. - Hoffmann, H., Platzer, C., von Münch, E., Winker, M. (2011): Technology review of constructed wetlands – Subsurface flow constructed wetlands for greywater and domestic wastewater treatment. Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH, Eschborn, Germany
- Timoshkin, O. A., ed. (2004). Index of animal species inhabiting Lake Baikal and its catchment area. Guides and Keys to Identification of Fauna and Flora of Lake Baikal. Vol. 1 (First ed.). Novosibirsk, Nauka: John Wiley & Sons. ISBN 978-5-02-031736-9.
- Johnson, W. C.; Millett, B. V.; Gilmanov, T.; Voldseth, R. A.; Guntenspergen, G. R. & Naugle, D. E. (2005). "Vulnerability of Northern Prairie Wetlands to Climate Change". Bio Science. 10: 863–872.
- Maltby, E. (1986). Waterlogged wealth: why waste the world's wet places?. Earthscan. London: International Institute for Environment and Development. ISBN 978-0905347639.
- "The Ramsar Information Sheet on Wetlands of International Importance". September 18, 2009. Retrieved November 19, 2011.
- The Food and Agriculture Organization (FAO): a specialized agency of the United Nations
- Synthesis of Adaptation Options for Coastal Areas. Climate Ready Estuaries Program, EPA 430-F-08-024. Washington, DC: US Environmental Protection Agency. 2009.
- "Coastal Wetland Protection". Project Drawdown. 2020-02-06. Retrieved 2020-09-13.
- Chmura, G. L. (2003). "Global carbon sequestration in tidal, saline wetland soils". Global Biogeochemical Cycles. 17 (4): 1111. Bibcode:2003GBioC..17.1111C. doi:10.1029/2002GB001917. S2CID 36119878.
- Roulet, N. T. (2000). "Peatlands, Carbon Storage, Greenhouse Gases, And The Kyoto Protocol: Prospects And Significance For Canada". Wetlands. 20 (4): 605–615. doi:10.1672/0277-5212(2000)020[0605:pcsgga]2.0.co;2. S2CID 7490212.
- "More on blue carbon and carbon sequestration".
- Van de Ven, G. P. (2004). Man-Made Lowlands: History of water management and land reclamation in the Netherlands. Utrecht: Uitgeverij Matrijs.
- Wells, Samuel A. (1830). A History of the Drainage of the Great Level of the Fens called Bedford Level 2. London: R. Pheney.
- Dahl, Thomas E.; Allord, Gregory J. "History of Wetlands in the Conterminous United States".
- Lander, Brian (2014). "State Management of River Dikes in Early China: New Sources on the Environmental History of the Central Yangzi Region". T'oung Pao. 100 (4–5): 325–362. doi:10.1163/15685322-10045p02.
- "unknown title". New Scientist. No. 1894. 1993-10-09. p. 46.
- "Good practices and lessons learned in integrating ecosystem conservation and poverty reduction objectives in wetlands". The Ramsar Convention on Wetlands. 2008-12-01. Retrieved 10 May 2022.
- Adamus, P. (2016). "Manual for the Wetland Ecosystem Services Protocol (WESP)" (PDF). Oregon State University. Retrieved July 28, 2018.
- Corbin, JD; Holl, KD (2012). "Applied nucleation as a forest restoration strategy". Forest Ecology and Management. 256: 37–46. doi:10.1016/j.foreco.2011.10.013.
- "EPA Regulations listed at 40 CFR 230.3(t)". US Environmental Protection Agency. March 2015. Retrieved 2014-02-18.
- US Government Publishing Office. (2011) 16 U.S. Code Chapter 58 Subchapter I, § 3801 – Definitions. Legal Information Institute, Cornell Law School, Ithaca.
- Rubec, Clayton DA; Hanson, Alan R (2009). "Wetland mitigation and compensation: Canadian experience". Wetlands Ecol Manage. 17: 3–14. doi:10.1007/s11273-008-9078-6. S2CID 32876048.