Carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.[1]

The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl).
The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond.
Carbonyl compounds
In organic chemistry, a carbonyl group characterizes the following types of compounds:
| Compound | Aldehyde | Ketone | Carboxylic acid | Carboxylate ester | Amide |
| Structure | 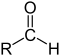 |  | .svg.png.webp) | ||
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | RCONR'R'' |
| Compound | Enone | Acyl halide | Acid anhydride | Imide |
| Structure | 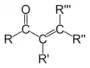 |  |  |  |
| General formula | RC(O)C(R')CR''R''' | RCOX | (RCO)2O | RC(O)N(R')C(O)R'' |

Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide.
A special group of carbonyl compounds are dicarbonyl compounds, which can exhibit special properties.
Structure and reactivity
For organic compounds, the length of the C-O bond does not vary widely from 120 picometers. Inorganic carbonyls have shorter C-O distances: CO, 113; CO2, 116; and COCl2, 116 pm.[2]
The carbonyl carbon is typically electrophilic. A qualitative order of electrophilicity is RCHO (aldehydes) > R2CO (ketones) > RCO2R' (esters) > RCONH2 (amides). A variety of nucleophiles attack, breaking the carbon-oxygen double bond.

The polarity of C=O bond also enhances the acidity of any adjacent C-H bonds. The pKa values of acetaldehyde and acetone are 16.7 and 19 respectively,[3]
Spectroscopy
- Infrared spectroscopy: the C=O double bond absorbs infrared light at wavenumbers between approximately 1600–1900 cm−1(5263 nm to 6250 nm). The exact location of the absorption is well understood with respect to the geometry of the molecule. This absorption is known as the "carbonyl stretch" when displayed on an infrared absorption spectrum.[4] In addition, the ultraviolet-visible spectra of propanone in water gives an absorption of carbonyl at 257 nm.[5]
- Nuclear magnetic resonance: the C=O double-bond exhibits different resonances depending on surrounding atoms, generally a downfield shift. The 13C NMR of a carbonyl carbon is in the range of 160–220 ppm.
See also
- Carbon–oxygen bond
- Organic chemistry
- Functional group
- Bridging carbonyl
- Electrophilic addition
References
- Saul Patai, ed. (1966). The Carbonyl Group. PATAI'S Chemistry of Functional Groups. Vol. 1. John Wiley & Sons. doi:10.1002/9780470771051. ISBN 9780470771051.Jacob Zabicky, ed. (1970). The Carbonyl Group. PATAI'S Chemistry of Functional Groups. Vol. 2. John Wiley & Sons. doi:10.1002/9780470771228. ISBN 9780470771228.
- G. Berthier, J. Serre (1966). "General and Theoretical Aspects of the Carbonyl Group". In Saul Patai (ed.). The Carbonyl Group. PATAI'S Chemistry of Functional Groups. Vol. 1. John Wiley & Sons. p. 1-77. doi:10.1002/9780470771051.ch1. ISBN 9780470771051.
- Ouellette, R.J. and Rawn, J.D. "Organic Chemistry" 1st Ed. Prentice-Hall, Inc., 1996: New Jersey. ISBN 0-02-390171-3
- Mayo D.W., Miller F.A and Hannah R.W “Course Notes On The Interpretation of Infrared and Raman Spectra” 1st Ed. John Wiley & Sons Inc, 2004: New Jersey. ISBN 0-471-24823-1.
- "Archived copy" (PDF). Archived from the original (PDF) on 2015-08-24. Retrieved 2015-07-11.
{{cite web}}: CS1 maint: archived copy as title (link)
Further reading
- L.G. Wade, Jr. Organic Chemistry, 5th ed. Prentice Hall, 2002. ISBN 0-13-033832-X
- The Frostburg State University Chemistry Department. Organic Chemistry Help (2000).
- Advanced Chemistry Development, Inc. IUPAC Nomenclature of Organic Chemistry (1997).
- William Reusch. tara VirtualText of Organic Chemistry (2004).
- Purdue Chemistry Department (retrieved Sep 2006). Includes water solubility data.
- William Reusch. (2004) Aldehydes and Ketones Retrieved 23 May 2005.
- ILPI. (2005) The MSDS Hyperglossary- Anhydride.