Homo erectus
Homo erectus (/ˌhoʊmoʊ əˈrɛktəs/; meaning "upright man") is an extinct species of archaic human from the Pleistocene, with its earliest occurrence about 2 million years ago.[2] Several human species, such as H. heidelbergensis and H. antecessor — with the former generally considered to have been the ancestor to Neanderthals, Denisovans, and modern humans — appear to have evolved from H. erectus.[3] Its specimens are among the first recognizable members of the genus Homo. H. erectus was the first human ancestor to spread throughout Eurasia, with a continental range extending from the Iberian Peninsula to Java. Asian populations of H. erectus may be ancestral to H. floresiensis[4] and possibly to H. luzonensis.[5] The last known population of H. erectus is H. e. soloensis from Java, around 117,000–108,000 years ago.[1]
| Homo erectus Temporal range: Early Pleistocene – Late Pleistocene[1] | |
|---|---|
_presented_at_Paleozoological_Museum_of_China.jpg.webp) | |
| Replica of the skull of Peking Man at the Paleozoological Museum of China | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Suborder: | Haplorhini |
| Infraorder: | Simiiformes |
| Family: | Hominidae |
| Subfamily: | Homininae |
| Tribe: | Hominini |
| Genus: | Homo |
| Species: | †H. erectus |
| Binomial name | |
| †Homo erectus (Dubois, 1893) | |
| Synonyms | |
| |
H. erectus had a more modern gait and body proportions, and was the first human species to have exhibited a flat face, prominent nose, and possibly sparse body hair coverage. Though brain size certainly exceeds that of ancestor species, capacity varied widely depending on the population. In earlier populations, brain development seems to cease early in childhood, suggesting that offspring were largely self-sufficient at birth, thus limiting cognitive development through life. H. erectus was an apex predator;[6] sites generally show consumption of medium to large animals, such as bovines or elephants, and suggest the development of predatory behaviour and coordinated hunting. H. erectus is associated with the Acheulean stone tool industry, and is postulated to have been the earliest human ancestor capable of using fire, hunting and gathering in coordinated groups, caring for injured or sick group members, and possibly seafaring and art (though examples of art are controversial, and are otherwise rudimentary and few and far between).
H. erectus males and females may have been roughly the same size as each other (i.e. exhibited reduced sexual dimorphism), which could indicate monogamy in line with general trends exhibited in primates. Size, nonetheless, ranged widely from 146–185 cm (4 ft 9 in – 6 ft 1 in) in height and 40–68 kg (88–150 lb) in weight. It is unclear if H. erectus was anatomically capable of speech, though it is postulated they communicated using some proto-language.
Taxonomy
Naming

Despite what English naturalist Charles Darwin had hypothesised in his 1871 book Descent of Man, many late-19th century evolutionary naturalists postulated that Asia, not Africa, was the birthplace of humankind as it is midway between Europe and America, providing optimal dispersal routes throughout the world (the Out of Asia theory). Among these was German naturalist Ernst Haeckel, who argued that the first human species evolved on the now-disproven hypothetical continent "Lemuria" in what is now Southeast Asia, from a species he termed "Pithecanthropus alalus" ("speechless apeman").[7] "Lemuria" had supposedly sunk below the Indian Ocean, so no fossils could be found to prove this. Nevertheless, Haeckel's model inspired Dutch scientist Eugène Dubois to journey to the Dutch East Indies. Because no directed expedition had ever discovered human fossils (the few known had all been discovered by accident), and the economy was strained by the Long Depression, the Dutch government refused to fund Dubois. In 1887, he enlisted in the Dutch East India Army as a medical officer, and was able to secure a post in 1887 in the Indies to search for his "missing link" in his spare time.[8] On Java, he found a skullcap in 1891 and a femur in 1892 (Java Man) dating to the late Pliocene or early Pleistocene at the Trinil site along the Solo River, which he named Pithecanthropus erectus ("upright apeman") in 1893. He attempted unsuccessfully to convince the European scientific community that he had found an upright-walking ape-man. Given few fossils of ancient humans had even been discovered at the time, they largely dismissed his findings as a malformed non-human ape.[9]
The significance of these fossils would not be realized until the 1927 discovery of what Canadian palaeoanthropologist Davidson Black called "Sinanthropus pekinensis" (Peking Man) at the Zhoukoudian cave near Beijing, China. Black lobbied across North America and Europe for funding to continue excavating the site,[10] which has since become the most productive H. erectus site in the world.[11] Continued interest in Java led to further H. erectus fossil discoveries at Ngandong (Solo Man) in 1931, Mojokerto (Java Man) in 1936, and Sangiran (Java Man) in 1937. The Sangiran site yielded the best preserved Java Man skull.[12] German paleoanthropologist Franz Weidenreich provided much of the detailed description of the Chinese specimens in several monographs. The original specimens were lost during the Second Sino-Japanese War after an attempt to smuggle them out of China for safekeeping. Only casts remain.
Similarities between Java Man and Peking Man led Ernst Mayr to rename both as Homo erectus in 1950. Throughout much of the 20th century, anthropologists debated the role of H. erectus in human evolution. Early in the century, due in part to the discoveries at Java and Zhoukoudian, the belief that modern humans first evolved in Asia was widely accepted. A few naturalists—Charles Darwin the most prominent among them—theorized that humans' earliest ancestors were African. Darwin had pointed out that chimpanzees and gorillas, humans' closest relatives, evolved and exist only in Africa.[13]
In 1949, the species was reported in Swartkrans Cave, South Africa, by South African paleoanthropologists Robert Broom and John Talbot Robinson, who described it as "Telanthropus capensis".[14] Homo fossils have also been reported from nearby caves, but their species designation has been a tumultuous discussion. A few North African sites have additionally yielded H. erectus remains, which at first were classified as "Atlantanthropus mauritanicus" in 1951.[15] Beginning in the 1970s, propelled most notably by Richard Leakey, more were being unearthed in East Africa predominantly at the Koobi Fora site, Kenya, and Olduvai Gorge, Tanzania.[16]
Archaic human fossils unearthed across Europe used to be assigned to H. erectus, but have since been separated as H. heidelbergensis as a result of namely British physical anthropologist Chris Stringer's work.[17]
Evolution
Hominin timeline | ||||||||||||||||||||
−10 — – −9 — – −8 — – −7 — – −6 — – −5 — – −4 — – −3 — – −2 — – −1 — – 0 — | Hominini Nakalipithecus Ouranopithecus Oreopithecus Sahelanthropus Orrorin Ardipithecus Homo bodoensis Homo sapiens Neanderthals,Denisovans |
| ||||||||||||||||||
(million years ago) | ||||||||||||||||||||
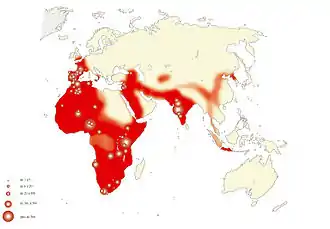
It has been proposed that H. erectus evolved from H. habilis about 2 Mya, though this has been called into question because they coexisted for at least a half a million years. Alternatively, a group of H. habilis may have been reproductively isolated, and only this group developed into H. erectus (cladogenesis).[18]
Because the earliest remains of H. erectus are found in both Africa and East Asia (in China as early as 2.1 Mya,[19][20][21] in South Africa 2.04 Mya[2][22]), it is debated where H. erectus evolved. A 2011 study suggested that it was H. habilis who reached West Asia from Africa, that early H. erectus developed there, and that early H. erectus would then have dispersed from West Asia to East Asia (Peking Man), Southeast Asia (Java Man), back to Africa (Homo ergaster), and to Europe (Tautavel Man), eventually evolving into modern humans in Africa.[23][24] Others have suggested that H. erectus/H. ergaster developed in Africa, where it eventually evolved into modern humans.[25][26]
H. erectus had reached Sangiran, Java, by 1.6 Mya, and a second and distinct wave of H. erectus had colonized Zhoukoudian, China, about 780 kya. Early teeth from Sangiran are bigger and more similar to those of basal (ancestral) Western H. erectus and H. habilis than to those of the derived Zhoukoudian H. erectus. However, later Sangiran teeth seem to reduce in size, which could indicate a secondary colonization event of Java by the Zhoukoudian or some closely related population.[27]
Subspecies
- Homo erectus erectus (Java Man, 1.6–0.5 Ma)
- Homo erectus ergaster (1.9–1.4 Ma)
- Homo erectus georgicus (1.8–1.6 Ma)
- Homo erectus lantianensis (Lantian Man, 1.6 Ma)
- Homo erectus nankinensis (Nanjing Man, 0.6 Ma)
- Homo erectus pekinensis (Peking Man, 0.7 Ma)
- Homo erectus soloensis (Solo Man, 0.546–0.143 Ma)
- Homo erectus tautavelensis (Tautavel Man, 0.45 Ma)
- Homo erectus yuanmouensis (Yuanmou Man)
"Wushan Man" was proposed as Homo erectus wushanensis, but is now thought to be based upon fossilized fragments of an extinct non-hominin ape.[28]
Since its discovery in 1893 (Java man), there has been a trend in palaeoanthropology of reducing the number of proposed species of Homo, to the point where H. erectus includes all early (Lower Paleolithic) forms of Homo sufficiently derived from H. habilis and distinct from early H. heidelbergensis (in Africa also known as H. rhodesiensis).[29] It is sometimes considered as a wide-ranging, polymorphous species.[30]
Due to such a wide range of variation, it has been suggested that the ancient H. rudolfensis and H. habilis should be considered early varieties of H. erectus.[31][32] The primitive H. e. georgicus from Dmanisi, Georgia has the smallest brain capacity of any known Pleistocene hominin (about 600 cc), and its inclusion in the species would greatly expand the range of variation of H. erectus to perhaps include species as H. rudolfensis, H. gautengensis, H. ergaster, and perhaps H. habilis.[33] However, a 2015 study suggested that H. georgicus represents an earlier, more primitive species of Homo derived from an older dispersal of hominins from Africa, with H. ergaster/erectus possibly deriving from a later dispersal.[34] H. georgicus is sometimes not even regarded as H. erectus.[35][36]
It is debated whether the African H. e. ergaster is a separate species (and that H. erectus evolved in Asia, then migrated to Africa),[37] or is the African form (sensu lato) of H. erectus (sensu stricto). In the latter, H. ergaster has also been suggested to represent the immediate ancestor of H. erectus.[38] It has also been suggested that H. ergaster instead of H. erectus, or some hybrid between the two, was the immediate ancestor of other archaic humans and modern humans. It has been proposed that Asian H. erectus have several unique characteristics from non-Asian populations (autapomorphies), but there is no clear consensus on what these characteristics are or if they are indeed limited to only Asia. Based on supposed derived characteristics, the 120 ka Javan H. e. soloensis has been proposed to have speciated from H. erectus, as H. soloensis, but this has been challenged because most of the basic cranial features are maintained.[39]
In a wider sense, H. erectus had mostly been replaced by H. heidelbergensis by about 300 kya, with possible late survival of H. erectus soloensis in Java an estimated 117-108kya.[1]

Descendants and synonyms
Homo erectus is the most long-lived species of Homo, having survived for almost two million years. By contrast, Homo sapiens emerged about a third of a million years ago.
Regarding many archaic humans, there is no definite consensus as to whether they should be classified as subspecies of H. erectus or H. sapiens or as separate species.
- African H. erectus candidates
- Homo ergaster ("African H. erectus")
- Homo naledi (or H. e. naledi)
- Eurasian H. erectus candidates:
- Homo antecessor (or H. e. antecessor)
- Homo heidelbergensis (or H. e. heidelbergensis)
- Homo cepranensis (or H. e. cepranensis)
- Homo floresiensis[40]
- Homo sapiens candidates
- Homo neanderthalensis (or H. s. neanderthalensis)
- Denisovans (or Homo sp. 'Denisova' H. sapiens ssp. 'Denisova' or H. sp. 'Altai')
- Homo rhodesiensis (or H. s. rhodesiensis)
- Homo heidelbergensis (or H. s. heidelbergensis)
- Homo sapiens idaltu
- the Narmada fossil, discovered in 1982 in Madhya Pradesh, India, was at first suggested as H. erectus (Homo erectus narmadensis) but later recognized as H. sapiens.[41]
Meganthropus, based on fossils found in Java, dated to between 1.4 and 0.9 Mya, was tentatively grouped with H. erectus in contrast to earlier interpretations of it as a giant species of early human[29] although older literature has placed the fossils outside of Homo altogether.[42] However, Zanolli et al. (2019) judged Meganthropus to be a distinct genus of extinct ape.[43]
Anatomy
Head

Homo erectus featured a flat face compared to earlier hominins; pronounced brow ridge; and a low, flat skull.[44][45] The presence of sagittal, frontal, and coronal keels, which are small crests that run along these suture lines, has been proposed to be evidence of significant thickening of the skull, specifically the cranial vault. CT scan analyses reveal this to not be the case. However, the squamous part of occipital bone, particularly the internal occipital crest, at the rear of the skull is notably thicker than that of modern humans, likely a basal (ancestral) trait.[45][46] The fossil record indicates that H. erectus was the first human species to have featured a projecting nose, which is generally thought to have evolved in response to breathing dry air in order to retain moisture.[47] American psychologist Lucia Jacobs hypothesized that the projecting nose instead allowed for distinguishing the direction different smells come from (stereo olfaction) to facilitate navigation and long-distance migration.[48]
The average brain size of Asian H. erectus is about 1,000 cc (61 cu in). However, markedly smaller specimens have been found in Dmanisi, Georgia (H. e. georgicus); Koobi Fora and Olorgesailie, Kenya; and possibly Gona, Ethiopia. Overall, H. erectus brain size varies from 546–1,251 cc (33.3–76.3 cu in),[49] which is greater than the range of variation seen in modern humans and chimps, though less than that of gorillas.
In an article published in 2021 titled 'Interpopulational variation in human brain size: implications for hominin cognitive phylogeny' it was found that the brain size of Asian H. erectus over the last 600,000 years overlaps significantly with modern human populations. Significantly, some small brained modern populations showed greater affinity with H. erectus than they did with other large brained and large bodied modern populations. The paper points out methodological flaws in current understanding of brain size increase in human evolution, where species averages are compared with fossils, which overlooks interpopulational variation. It also overlooks the fact that some modern populations have not seen any dramatic brain size increase relative to H. erectus with most of the increase occurring in European populations, which has the result of obscuring interpopulational variation. As the authors write '...the increase in the mean of H. sapiens cranial capacity is to a large extent due to an increase in the upper limit with a much less pronounced increase in the lower limit relative to our H. erectus sample. And this increase in the upper limit seems to be more pronounced in European populations – which may be a result of correlated increases in body size in addition to climatic factors'. Consequently, the authors argue that purely based on brain size similarities, Asian H. erectus could be re-classified as a subspecies of H. sapiens, that is H. sapiens soloensis - as was suggested by earlier authors.[50]
Dentally, H. erectus have the thinnest enamel of any Plio–Pleistocene hominin. Enamel prevents the tooth from breaking from hard foods, but impedes shearing through tough foods. The bodies of the mandibles of H. erectus, and all early Homo, are thicker than those of modern humans and all living apes. The mandibular body resists torsion from the bite force or chewing, meaning their jaws could produce unusually powerful stresses while eating, but the practical application of this is unclear. Nonetheless, the mandibular bodies of H. erectus are somewhat thinner than those of early Homo. The premolars and molars also have a higher frequency of pits than H. habilis, suggesting H. erectus ate more brittle foods (which cause pitting). These all indicate that the H. erectus mouth was less capable of processing hard foods and more at shearing through tougher foods, thus reducing the variety of foods it could process, likely as a response to tool use.[51]
Body
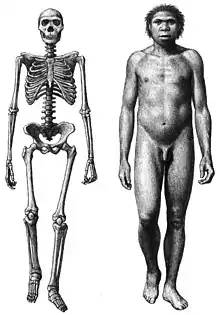
Like modern humans, H. erectus varied widely in size, ranging from 146–185 cm (4 ft 9 in – 6 ft 1 in) in height and 40–68 kg (88–150 lb) in weight, thought to be due to regional differences in climate, mortality rates, or nutrition.[52][53] Among primates, this marked of a response to environmental stressors (phenotypic plasticity) is only demonstrated in modern humans.[54][55][56]
Like modern humans and unlike other great apes, there does not seem to have been a great size disparity between H. erectus males and females (size-specific sexual dimorphism), though there is not much fossil data regarding this.[57] Brain size in two adults from Koobi Fora measured 848 and 804 cc (51.7 and 49.1 cu in),[49] and another significantly smaller adult measured 691 cc (42.2 cu in), which could possibly indicate sexual dimorphism, though sex was undetermined.[18] Another case that depicts the difficulty of assigning sex to the fossil record is a few samples taken in Olduvai Gorge. In 1960, in Olduvai Gorge two skulls identified as OH12 and OH9, were found to be that of H. erectus with a cranial capacities of 1000cc and 700cc.[58] It is unclear if sexual dimorphism is at play here since the remains are fragmentary.[58] If H. erectus did not exhibit sexual dimorphism, then it is possible that they were the first in the human line to do so, though the fragmentary fossil record for earlier species makes this unclear. If yes, then there was a substantial and sudden increase in female height.[59] Certain features of sexual dimorphism are often identified in the possibility of determining sex such as lack of muscle marking.[60]

H. erectus had about the same limb configurations and proportions as modern humans, implying humanlike locomotion,[61] the first in the Homo lineage.[54] H. erectus tracks near Ileret, Kenya, also indicate a human gait.[62] A humanlike shoulder suggests an ability for high speed throwing.[63] It was once thought that Turkana boy had 6 lumbar vertebra instead of the 5 seen in modern humans and 11 instead of 12 thoracic vertebrae, but this has since been revised, and the specimen is now considered to have exhibited a humanlike curvature of the spine (lordosis) and the same number of respective vertebrae.[64]
It is largely unclear when human ancestors lost most of their body hair. Genetic analysis suggests that high activity in the melanocortin 1 receptor, which would produce dark skin, dates back to 1.2 Mya. This could indicate the evolution of hairlessness around this time, as a lack of body hair would have left the skin exposed to harmful UV radiation.[65] It is possible that exposed skin only became maladaptive in the Pleistocene, because the increasing tilt of the Earth (which also caused the ice ages) would have increased solar radiation bombardment- which would suggest that hairlessness first emerged in the australopithecines.[66] However, australopithecines seem to have lived at much higher, much colder elevations—typically 1,000–1,600 m (3,300–5,200 ft) where the nighttime temperature can drop to 10 or 5 °C (50 or 41 °F)—so they may have required hair to stay warm, unlike early Homo which inhabited lower, hotter elevations.[67] Populations in higher latitudes potentially developed lighter skin to prevent vitamin D deficiency.[68] A 500–300 ka H. erectus specimen from Turkey was diagnosed with the earliest known case of tuberculous meningitis, which is typically exacerbated in dark-skinned people living in higher latitudes due to vitamin D deficiency.[69] Hairlessness is generally thought to have facilitated sweating,[70] but reduction of parasite load and sexual selection have also been proposed.[71][72]
Metabolism

The 1.8 Ma Mojokerto child specimen from Java, who died at about 1 year of age, presented 72–84% of the average adult brain size, which is more similar to the faster brain growth trajectory of great apes than modern humans. This indicates that H. erectus was probably not cognitively comparable to modern humans, and that secondary altriciality—an extended childhood and long period of dependency due to the great amount of time required for brain maturation—evolved much later in human evolution, perhaps in the modern human/Neanderthal last common ancestor.[73] It was previously believed that, based on the narrow pelvis of Turkana boy, H. erectus could only safely deliver a baby with a brain volume of about 230 cc (14 cu in), equating to a similar brain growth rate as modern humans to achieve the average adult brain size of 600–1,067 cc (36.6–65.1 cu in). However, a 1.8 Ma female pelvis from Gona, Ethiopia, shows that H. erectus babies with a brain volume of 310 cc (19 cu in) could have been safely delivered, which is 34–36% the mean adult size, compared to 40% in chimps and 28% in modern humans. This more aligns with the conclusions drawn from the Mojokerto child.[57] A faster development rate could indicate a lower expected lifespan.[74]
Based on an average mass of 63 kg (139 lb) for males and 52.3 kg (115 lb) for females, the total energy expenditure (TEE)—the amount of calories consumed in one day—was estimated to be about 2271.8 and 1909.5 kcal, respectively. This is similar to that of earlier Homo, despite a marked increase in activity and migratory capacity, likely because the longer legs of H. erectus were more energy-efficient in long-distance movement. Nonetheless, the estimate for H. erectus females is 84% higher than that for Australopithecus females, possibly due to an increased body size and a decreased growth rate.[75] A 2011 study, assuming high energy or dietary fat requirements based on the abundance of large game animals at H. erectus sites, calculated a TEE of 2,700–3,400 kcal of which 27–44% derived from fat, and 44–62% of the fat from animal sources. In comparison, modern humans with a similar activity level have a DEE of 2,450 calories, of which 33% derives from fat, and 49% of the fat from animals.[76]
Bone thickness

The cortical bone (the outer layer of the bone) is extraordinarily thickened, particularly in East Asian populations. The skullcaps have oftentimes been confused with fossil turtle carapaces,[77] and the medullary canal in the long bones (where the bone marrow is stored, in the limbs) is extremely narrowed (medullary stenosis). This degree of thickening is usually exhibited in semi-aquatic animals which used their heavy (pachyosteosclerotic) bones as ballasts to help them sink, induced by hypothyroidism. Male specimens have thicker cortical bone than females.[78]
It is largely unclear what function this could have served. All pathological inducers would leave scarring or some other indicator not normally exhibited in H. erectus. Before more complete skeletons were discovered, Weidenreich suggested H. erectus was a gigantic species, thickened bone required to support the massive weight. It was hypothesised that intense physical activity could have induced bone thickening, but in 1970, human biologist Stanley Marion Garn demonstrated there is a low correlation between the two at least in modern humans. Garn instead noted different races have different average cortical bone thicknesses, and concluded it is genetic rather than environmental. It is unclear if the condition is caused by increased bone apposition (bone formation) or decreased bone resorption, but Garn noted the stenosis is quite similar to the congenital condition in modern humans induced by hyper-apposition. In 1985, biological anthropologist Gail Kennedy argued for resorption as a result of hyperparathyroidism caused by hypocalcemia (calcium deficiency), a consequence of a dietary shift to low-calcium meat. Kennedy could not explain why the calcium metabolism of H. erectus never adjusted.[78] In 1985, American palaeoanthropologist Mary Doria Russell and colleagues argued the supraorbital torus is a response to withstanding major bending stress which localises in that region when significant force is applied through the front teeth, such as while using the mouth as a third hand to carry objects.[79]
In 2004, Noel Boaz and Russel Ciochon suggested it was a result of a cultural practice, wherein H. erectus would fight each other with fists, stones, or clubs to settle disputes or battle for mates, since the skull is reinforced in key areas. The mandible is quite robust, capable of absorbing heavy blows (no "glass jaw"); the heavy brow ridge protects the eyes, and transitions into a bar covering the ears, connecting all the way in the back of the skull, meaning blows to any of these regions can be effectively dissipated across the skull; and the sagittal keel protects the top of the braincase. Many skullcaps bear usually debilitating fractures, such as the Peking Man skull X, yet they can show signs of surviving and healing. Anthropologist Peter Brown suggested a similar reason for the unusual thickening of the modern Australian Aboriginal skull, a result of a ritual popular in central and southeast Australian tribes where adversaries would wack each other with waddies (sticks) until knockout.[77]
Culture
Social structure

The only fossil evidence regarding H. erectus group composition comes from 4 sites outside of Ileret, Kenya, where 97 footprints made 1.5 Mya were likely left by a group of at least 20 individuals. One of these trackways, based on the size of the footprints, may have been an entirely male group, which could indicate they were some specialised task group, such as a hunting or foraging party, or a border patrol. If correct, this would also indicate sexual division of labour, which distinguishes human societies from those of other great apes and social mammalian carnivores. In modern hunter gatherer societies who target large prey items, typically male parties are dispatched to bring down these high-risk animals, and, due to the low success rate, female parties focus on more predictable foods.[62] Based on modern day savanna chimp and baboon group composition and behaviour, H. erectus ergaster may have lived in large, multi-male groups in order to defend against large savanna predators in the open and exposed environment.[80] However, dispersal patterns indicate that H. erectus generally avoided areas with high carnivore density.[81] It is possible that male–male bonding and male–female friendships were important societal aspects.[80]
Because H. erectus children had faster brain growth rates, H. erectus likely did not exhibit the same degree of maternal investment or child-rearing behaviours as modern humans.[57]
Because H. erectus males and females are thought to have been about the same size compared to other great apes (exhibit less size-specific sexual dimorphism), it is generally hypothesised that they lived in a monogamous society, as reduced sexual dimorphism in primates is typically correlated with this mating system.[59] However, it is unclear if H. erectus did in fact exhibit humanlike rates of sexual dimorphism.[18] If they did, then it would mean only female height increased from the ancestor species, which could have been caused by a shift in female fertility or diet, and/or reduced pressure on males for large size. This in turn could imply a shift in female behaviour which made it difficult for males to maintain a harem.[82]
Food
Increasing brain size is often directly associated with a meatier diet and resultant higher caloric intake. An increase in insect protein consumption, Entomophagy, has also been proposed as a possible cause. However, it is also possible that the energy-expensive guts decreased in size in H. erectus, because the large ape gut is used to synthesize fat by fermenting plant matter which was replaced by dietary animal fat, allowing more energy to be diverted to brain growth. This would have increased brain size indirectly while maintaining the same caloric requirements of ancestor species. H. erectus may have also been the first to use a hunting and gathering food collecting strategy as a response to the increasing dependence on meat. With an emphasis on teamwork, division of labor, and food sharing, hunting and gathering was a dramatically different subsistence strategy from previous modes.[51][76]

H. erectus sites frequently are associated with assemblages of medium- to large-sized game, namely elephants, rhinos, hippos, bovine, and boar. H. erectus would have had considerable leftovers, potentially pointing to food sharing or long-term food preservation (such as by drying) if most of the kill was indeed utilized. It is possible that H. erectus grew to become quite dependent on large-animal meat, and the disappearance of H. erectus from the Levant is correlated with the local extinction of the straight-tusked elephant.[76] Nonetheless, H. erectus diet likely varied widely depending upon location. For example, at the 780 ka Gesher Benot Ya'aqov site, Israel, the inhabitants gathered and ate 55 different types of fruits, vegetables, seeds, nuts, and tubers, and it appears that they used fire to roast certain plant materials that otherwise would have been inedible; they also consumed amphibians, reptiles, birds, aquatic and terrestrial invertebrates, in addition to the usual large creatures such as elephant and fallow deer.[83] At the 1.95 Ma FwJJ20 lakeside site in the East Turkana Basin, Kenya, the inhabitants ate (alongside the usual bovids, hippos, and rhinos) aquatic creatures such as turtles, crocodiles, and catfish. The large animals were likely scavenged at this site, but the turtles and fish were possibly collected live.[84] At the 1.5 Ma Trinil H. K. site, Java, H. erectus likely gathered fish and shellfish.[85]
Dentally, H. erectus mouths were not as versatile as those of ancestor species, capable of processing a narrower range of foods. However, tools were likely used to process hard foods, thus affecting the chewing apparatus, and this combination may have instead increased dietary flexibility (though this does not equate to a highly varied diet). Such versatility may have permitted H. erectus to inhabit a range of different environments, and migrate beyond Africa.[51]
In 1999, British anthropologist Richard Wrangham proposed the "cooking hypothesis" which states that H. erectus speciated from the ancestral H. habilis because of fire usage and cooking 2 Million years ago to explain the rapid doubling of brain size between these two species in only a 500,000 year timespan, and the sudden appearance of the typical human body plan. Cooking makes protein more easily digestible, speeds up nutrient absorption, and destroys food-borne pathogens, which would have increased the environment's natural carrying capacity, allowing group size to expand, causing selective pressure for sociality, requiring greater brain function.[86][87] However, the fossil record does not associate the emergence of H. erectus with fire usage nor with any technological breakthrough for that matter, and cooking likely did not become a common practice until after 400 kya.[51][76]
Java Man's dispersal through Southeast Asia coincides with the extirpation of the giant turtle Megalochelys, possibly due to overhunting as the turtle would have been an easy, slow-moving target which could have been stored for quite some time.[88]
Tool production


H. erectus is credited with inventing the Acheulean stone tool industry, succeeding the Oldowan industry,[89][90] and were the first to make lithic flakes bigger than 10 cm (3.9 in), and hand axes (which includes bifacial tools with only 2 sides, such as picks, knives, and cleavers).[91] Though larger and heavier, these hand axes had sharper, chiseled edges.[92] They were likely multi-purpose tools, used in variety of activities such as cutting meat, wood, or edible plants.[93] In 1979, American paleontologist Thomas Wynn stated that Acheulean technology required operational intelligence (foresight and planning), being markedly more complex than Oldowan technology which included lithics of unstandardized shape, cross-sections, and symmetry. Based on this, he concluded that there is not a significant disparity in intelligence between H. erectus and modern humans and that, for the last 300,000 years, increasing intelligence has not been a major influencer of cultural evolution.[94] However, a 1 year old H. erectus specimen shows that this species lacked an extended childhood required for greater brain development, indicating lower cognitive capabilities.[73] A few sites, likely due to occupation over several generations, features hand axes en masse, such as at Melka Kunture, Ethiopia; Olorgesailie, Kenya; Isimila, Tanzania; and Kalambo Falls, Zambia.[93]
The earliest record of Acheulean technology comes from West Turkana, Kenya 1.76 Mya. Oldowan lithics are also known from the site, and the two seemed to coexist for some time. The earliest records of Acheulean technology outside of Africa date to no older than 1 Mya, indicating it only became widespread after some secondary H. erectus dispersal from Africa.[92]
On Java, H. erectus produced tools from shells at Sangiran[95] and Trinil.[96] Spherical stones, measuring 6–12 cm (2.4–4.7 in) in diameter, are frequently found in African and Chinese Lower Paleolithic sites, and were potentially used as bolas; if correct, this would indicate string and cordage technology.[97]
Fire
H. erectus is credited as the first human ancestor to have used fire, though the timing of this invention is debated mainly because campfires very rarely and very poorly preserve over long periods of time, let alone thousands or millions of years. The earliest claimed fire sites are in Kenya, FxJj20 at Koobi Fora[98][86][99] and GnJi 1/6E in the Chemoigut Formation, as far back as 1.5 Mya,[86][99] and in South Africa, Wonderwerk Cave, 1.7 Mya.[100] The first firekeepers are thought to have simply transported to caves and maintained naturally occurring fires for extended periods of time or only sporadically when the opportunity arose. Maintaining fires would require firekeepers to have knowledge on slow-burning materials such as dung.[86] Fire becomes markedly more abundant in the wider archaeological record after 400,000–300,000 years ago, which can be explained as some advancement in fire management techniques took place at this time[86] or human ancestors only opportunistically used fire until this time.[99][101][51][76] It is possible that firestarting was invented and lost and reinvented multiple times and independently by different communities rather than being invented in one place and spreading throughout the world.[101] The earliest evidence of hearths comes from Gesher Benot Ya'aqov, Israel, over 700,000 years ago, where fire is recorded in multiple layers in an area close to water, both uncharacteristic of natural fires.[87]
Artificial lighting may have led to increased waking hours—modern humans have about a 16-hour waking period, whereas other apes are generally awake from only sunup to sundown—and these additional hours were probably used for socializing. Because of this, fire usage is probably also linked to the origin of language.[86][87] Artificial lighting may have also made sleeping on the ground instead of the trees possible by keeping terrestrial predators at bay.[87]
Migration into the frigid climate of Ice Age Europe may have only been possible because of fire, but evidence of fire usage in Europe until about 400–300,000 years ago is notably absent.[99] If these early European H. erectus did not have fire, it is largely unclear how they stayed warm, avoided predators, and prepared animal fat and meat for consumption; and lightning is less common farther north equating to a reduced availability of naturally occurring fires. It is possible that they only knew how to maintain fires in certain settings in the landscapes and prepared food some distance away from home, meaning evidence of fire and evidence of hominin activity are spaced far apart.[87] Alternatively, H. erectus may have only pushed farther north during warmer interglacial periods—thus not requiring fire, food storage, or clothing technology—[102] and their dispersal patterns indicate they generally stayed in warmer lower-to-middle latitudes.[81] It is debated if the H. e. pekinensis inhabitants of Zhoukoudian, Northern China, were capable of controlling fires as early as 770 kya to stay warm in what may have been a relatively cold climate.[103]
Construction

In 1962, a 366 cm × 427 cm × 30 cm (12 ft × 14 ft × 1 ft) circle made with volcanic rocks was discovered in Olduvai Gorge. At 61–76 cm (2–2.5 ft) intervals, rocks were piled up to 15–23 cm (6–9 in) high. British palaeoanthropologist Mary Leakey suggested the rock piles were used to support poles stuck into the ground, possibly to support a windbreak or a rough hut. Some modern day nomadic tribes build similar low-lying rock walls to build temporary shelters upon, bending upright branches as poles and using grasses or animal hide as a screen.[105] Dating to 1.75 Mya, it is the oldest claimed evidence of architecture.[106]
In Europe, evidence of constructed dwelling structures dating to or following the Holstein Interglacial (which began 424 kya) has been claimed in Bilzingsleben, Germany; Terra Amata, France; and Fermanville and Saint-Germain-des-Vaux in Normandy. The oldest evidence of a dwelling (and a campfire) in Europe comes from Přezletice, Czech Republic, 700 kya during the Cromerian Interglacial. This dwelling's base measured about 3 m × 4 m (9.8 ft × 13.1 ft) on the exterior and 3 m × 2 m (9.8 ft × 6.6 ft) on the interior, and is considered to have been a firm surface hut, probably with a vaulted roof made of thick branches or thin poles, supported by a foundation of big rocks and earth, and likely functioned as a winter base camp.[107]
The earliest evidence of cave habitation is Wonderwerk Cave, South Africa, about 1.6 Mya, but evidence of cave use globally is sporadic until about 600 kya.[108]
Clothing
_Fundort_Nariokotome%252C_Kenia%252C_Rekonstruktion_im_Neanderthal_Museum.jpg.webp)
It is largely unclear when clothing was invented, with the earliest estimate stretching as far back as 3 Mya to compensate for a lack of insulating body hair.[66] It is known that head lice and body lice (the latter can only inhabit clothed individuals) for modern humans diverged about 170 kya, well before modern humans left Africa, meaning clothes were already well in use before encountering cold climates. One of the first uses of animal hide is thought to have been for clothing, and the oldest hide scrapers date to about 780 kya, though this is not indicative of clothing.[109]
Seafaring
Acheulean artifacts discovered on isolated islands that were never connected to land in the Pleistocene may show seafaring by H. erectus as early as 1 Mya in Indonesia. They had arrived on the islands of Flores, Timor, and Roti, which would have necessitated crossing the Lombok Strait (the Wallace Line), at least before 800 kya. It is also possible they were the first European mariners as well and crossed the Strait of Gibraltar between North Africa and Spain. A 2021 genetic analysis of these island populations of H. erectus found no evidence of interbreeding with modern humans.[110] Seafaring capability would show H. erectus had a great capacity for planning, likely months in advance of the trip.[111][112]
Similarly, Homo luzonensis is dated between 771,000 to 631,000 years ago. Because Luzon has always been an island in the Quaternary, the ancestors of H. luzonensis would have had to have made a substantial sea crossing and crossed the Huxley Line.[113]
Healthcare
.jpg.webp)
The earliest probable example of infirming sick group members is a 1.77 Ma H. e. georgicus specimen who had lost all but one tooth due to age or gum disease, the earliest example of severe chewing impairment, yet still survived for several years afterwards. However, it is possible australopithecines were capable of caring for debilitated group members.[114] Unable to chew, this H. e. georgicus individual probably ate soft plant or animal foods possibly with assistance from other group members. High-latitude groups are thought to have been predominantly carnivorous, eating soft tissue such as bone marrow or brains, which may have increased survival rates for toothless individuals.[115]
The 1.5 Ma Turkana boy was diagnosed with juvenile spinal disc herniation, and, because this specimen was still growing, this caused some scoliosis (abnormal curving of the spine). These usually cause recurrent lower back pain and sciatica (pain running down the leg), and likely restricted Turkana boy in walking, bending, and other daily activities. The specimen appears to have survived into adolescence, which evidences advanced group care.[116]
The 1,000–700 ka Java man specimen presents a noticeable osteocyte on the femur, likely Paget's disease of bone, and osteopetrosis, thickening of the bone, likely resulting from skeletal fluorosis caused by ingestion of food contaminated by fluorine-filled volcanic ash (as the specimen was found in ash-filled strata). Livestock that grazes on volcanic ash ridden fields typically die of acute intoxication within a few days or weeks.[117]
Art and rituals
.jpg.webp)

An engraved Pseudodon shell DUB1006-fL with geometric markings could possibly be evidence of the earliest art-making, dating back to 546–436 kya. Art-making capabilities could be considered evidence of symbolic thinking, which is associated with modern cognition and behavior.[96][118][119][120] In 1976, American archeologist Alexander Marshack asserted that engraved lines on an ox rib, associated with Acheulean lithics, from Pech de l'Azé, France, are similar to a meander design found in modern human Upper Paleolithic cave art.[121] Three ostrich eggshell beads associated with Achuelian lithics were found in northwestern Africa, the earliest disc beads ever found, and Acheulian disc beads have also been found in France and Israel.[111] The Middle Pleistocene "Venus of Tan-Tan" and "Venus of Berekhat Ram" are postulated to been crafted by H. erectus to resemble a human form. They were mostly formed by natural weathering, but slightly modified to emphasize certain grooves to suggest hairline, limbs, and eyes.[122][123] The former has traces of pigments on the front side, possibly indicating it was colored.[122]
H. erectus was also the earliest human to have intentionally collected red-colored pigments, namely ochre, recorded as early as the Middle Pleistocene. Ochre lumps at Olduvai Gorge, Tanzania—associated with the 1.4 Ma Olduvai Hominid 9—and Ambrona, Spain—which dates to 424–374 kya—were suggested to have been struck by a hammerstone and purposefully shaped and trimmed.[124][121] At Terra Amata, France—which dates to 425–400 or 355–325 kya—red, yellow, and brown ochres were recovered in association with pole structures; ochre was probably heated to achieve such a wide color range.[124][125] As it is unclear if H. erectus could have used ochre for any practical application, ochre collection might indicate that H. erectus was the earliest human to have exhibited a sense of aesthetics and to think beyond simply survival. Later human species are postulated to have used ochre as body paint, but in the case of H. erectus, it is contested if body paint was used so early in time. Further, it is unclear if these few examples are not simply isolated incidents of ochre use, as ochre is much more prevalent in Middle and Upper Paleolithic sites attributed to Neanderthals and H. sapiens.[126][121]
In 1935, Jewish German anthropologist Franz Weidenreich speculated that the inhabitants of the Chinese Zhoukoudian Peking Man site were members of some Lower Paleolithic Skull Cult because the skulls all showed fatal blows to the head, breaking in of the foramen magnum at the base of the skull, by-and-large lack of preserved facial aspects, an apparently consistent pattern of breaking on the mandible, and a lack of post-cranial remains (elements that are not the skull). He believed that the inhabitants were headhunters, and smashed open the skulls and ate the brains of their victims.[127][121] However, scavenging animals and natural forces such as flooding can also inflict the same kind of damage to skulls,[121] and there is not enough evidence to suggest manhunting or cannibalism.[128]
In 1999, British science writers Marek Kohn and Steven Mithen said that many hand axes exhibit no wear and were produced en masse, and concluded that these symmetrical, tear-drop shaped lithics functioned primarily as display tools so males could prove their fitness to females in some courting ritual, and were discarded afterwards.[129] However, an apparent lack of reported wearing is likely due to a lack of use-wear studies, and only a few sites yield an exorbitant sum of hand axes likely due to gradual accumulation over generations instead of mass production.[93]
Language
In 1984, the vertebral column of the 1.6 Ma adolescent Turkana boy indicated that this individual did not have properly developed respiratory muscles in order to produce speech. In 2001, American anthropologists Bruce Latimer and James Ohman concluded that Turkana boy was afflicted by skeletal dysplasia and scoliosis.[130] In 2006, American anthropologist Marc Meyer and colleagues described a 1.8 Ma H. e. georgicus specimen as having a spine within the range of variation of modern human spines, contending that Turkana boy had spinal stenosis and was thus not representative of the species. Also, because he considered H. e. georgicus ancestral to all non-African H. erectus, Meyer concluded that the respiratory muscles of all H. erectus (at least non-ergaster) would not have impeded vocalisation or speech production.[131] However, in 2013 and 2014, anthropologist Regula Schiess and colleagues concluded that there is no evidence of any congenital defects in Turkana boy, and considered the specimen representative of the species.[132][133]
Neurologically, all Homo have similarly configured brains, and, likewise, the Broca's and Wernicke's areas (in charge of sentence formulation and speech production in modern humans) of H. erectus were comparable to those of modern humans. However, this is not indicative of anything in terms of speech capability as even large chimpanzees can have similarly expanded Broca's area, and it is unclear if these areas served as language centers in archaic humans.[134] A 1-year-old H. erectus specimen shows that an extended childhood to allow for brain growth, which is a prerequisite in language acquisition, was not exhibited in this species.[73]
The hyoid bone supports the tongue and makes possible modulation of the vocal tract to control pitch and volume. A 400 ka H. erectus hyoid bone from Castel di Guido, Italy, is bar-shaped—more similar to that of other Homo than to that of non-human apes and Australopithecus—but is devoid of muscle impressions, has a shield-shaped body, and is implied to have had reduced greater horns, meaning H. erectus lacked a humanlike vocal apparatus and thus anatomical prerequisites for a modern human level of speech.[135] Increasing brain size and cultural complexity in tandem with technological refinement, and the hypothesis that articulate Neanderthals and modern humans may have inherited speech capabilities from the last common ancestor, could possibly indicate that H. erectus used some proto-language and built the basic framework which fully fledged languages would eventually be built around.[136] However, this ancestor may have instead been H. heidelbergensis, as a hyoid bone of a 530 ka H. heidelbergensis specimen from the Spanish Sima de los Huesos Cave is like that of modern humans,[137] and another specimen from the same area shows an auditory capacity sensitive enough to pick up human speech.[138]
Extinction
The last known occurrence of Homo erectus is 117,000–108,000 years ago in Ngandong, Java according to a study published in 2019.[1]
In 2020 researchers reported that Homo erectus and Homo heidelbergensis lost more than half of their climate niche – climate they were adapted to –, with no corresponding reduction in physical range, just before extinction and that climate change played a substantial role in extinctions of past Homo species.[139][140][141]
Fossils

The lower cave of the Zhoukoudian cave, China, is one of the most important archaeological sites worldwide.[142] There have been remains of 45 homo erectus individuals found and thousands of tools recovered.[142] Most of these remains were lost during World War 2, with the exception of two postcranial elements that were rediscovered in China in 1951 and four human teeth from 'Dragon Bone Hill'.[142]
New evidence has shown that Homo erectus does not have uniquely thick vault bones, as was previously thought.[143] Testing showed that neither Asian or African Homo erectus had uniquely large vault bones.[143]
Individual fossils
Some of the major Homo erectus fossils:
- Indonesia (island of Java): Trinil 2 (holotype), Sangiran collection, Sambungmachan collection,[144] Ngandong collection
- China ("Peking Man"): Lantian (Gongwangling and Chenjiawo), Yunxian, Zhoukoudian, Nanjing, Hexian
- Kenya: KNM ER 3883, KNM ER 3733
- Vietnam: Northern, Tham Khuyen,[145] Hoa Binh
- Republic of Georgia: Dmanisi collection ("Homo erectus georgicus")
- Ethiopia: Daka calvaria
- Eritrea: Buia cranium (possibly H. ergaster)[146]
- Denizli Province, Turkey: Kocabas fossil[69]
- Drimolen, South Africa: DNH 134[147]
Phylogeny
A cladogram of Homo erectus is as follows.[148] It is indicated how many million years ago the clades diverged.
| Homo (2.85) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Homo erectus was originally African. The extant Homo heidelbergensis (cladistically granting Homo sapiens), which was originally African, emerged within the Asian Homo erectus. Contemporary groups appear to have been interbreeding, so any phylogeny like this only gives a coarse impression of the evolution of Homo, and extinct lineage may have partially continued in other groupings. Not included are other contemporary groups such as Homo floresiensis, Homo naledi, Homo luzonensis, Homo rudolfensis, Australopithecus sediba, Australopithecus africanus, and Paranthropus.
Gallery
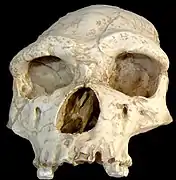 Homo erectus tautavelensis skull.
Homo erectus tautavelensis skull. Replica of lower jaws of Homo erectus from Tautavel, France.
Replica of lower jaws of Homo erectus from Tautavel, France..jpg.webp) Calvaria "Sangiran II" original, collection Koenigswald, Senckenberg Museum.
Calvaria "Sangiran II" original, collection Koenigswald, Senckenberg Museum. A reconstruction based on evidence from the Daka Member, Ethiopia
A reconstruction based on evidence from the Daka Member, Ethiopia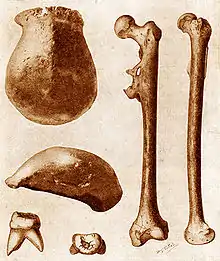 Original fossils of Pithecanthropus erectus (now Homo erectus) found in Java in 1891.
Original fossils of Pithecanthropus erectus (now Homo erectus) found in Java in 1891.
See also
- Anthropopithecus
- Kozarnika
- Multiregional origin of modern humans
General:
- List of fossil sites (with link directory)
- List of human evolution fossils (with images)
References
- Rizal Y, Westaway KE, Zaim Y, van den Bergh GD, Bettis EA, Morwood MJ, et al. (January 2020). "Last appearance of Homo erectus at Ngandong, Java, 117,000-108,000 years ago". Nature. 577 (7790): 381–385. doi:10.1038/s41586-019-1863-2. PMID 31853068. S2CID 209410644.
- Herries AI, Martin JM, Leece AB, Adams JW, Boschian G, Joannes-Boyau R, et al. (April 2020). "Contemporaneity of Australopithecus, Paranthropus, and early Homo erectus in South Africa". Science. 368 (6486): eaaw7293. doi:10.1126/science.aaw7293. PMID 32241925.
- Dembo M, Radovčić D, Garvin HM, Laird MF, Schroeder L, Scott JE, et al. (August 2016). "The evolutionary relationships and age of Homo naledi: An assessment using dated Bayesian phylogenetic methods". Journal of Human Evolution. 97: 17–26. doi:10.1016/j.jhevol.2016.04.008. hdl:2164/8796. PMID 27457542.
- van den Bergh GD, Kaifu Y, Kurniawan I, Kono RT, Brumm A, Setiyabudi E, et al. (June 2016). "Homo floresiensis-like fossils from the early Middle Pleistocene of Flores". Nature. 534 (7606): 245–248. Bibcode:2016Natur.534..245V. doi:10.1038/nature17999. PMID 27279221.
- Détroit F, Mijares AS, Corny J, Daver G, Zanolli C, Dizon E, et al. (April 2019). "A new species of Homo from the Late Pleistocene of the Philippines" (PDF). Nature. 568 (7751): 181–186. Bibcode:2019Natur.568..181D. doi:10.1038/s41586-019-1067-9. PMID 30971845. S2CID 106411053.
- Ben-Dor M, Sirtoli R, Barkai R (August 2021). "The evolution of the human trophic level during the Pleistocene". American Journal of Physical Anthropology. 175 (Suppl 72): 27–56. doi:10.1002/ajpa.24247. PMID 33675083.
- Theunissen 2012, p. 6.
- Theunissen 2012, p. 33.
- Yen HP (2014). "Evolutionary Asiacentrism, Peking man, and the origins of sinocentric ethno-nationalism". Journal of the History of Biology. 47 (4): 585–625. doi:10.1007/s10739-014-9381-4. PMID 24771020. S2CID 23308894.
- Sigmon 1981, p. 64.
- Yang L (2014). "Zhoukoudian: Geography and Culture". Encyclopedia of Global Archaeology. Springer Science+Business Media. pp. 7961–7965. doi:10.1007/978-1-4419-0465-2_1899. ISBN 978-1-4419-0466-9.
- Ciochon RL, Huffman OF (2014). "Java Man". In Smith C (ed.). Encyclopedia of Global Archaeology. pp. 4182–4188. doi:10.1007/978-1-4419-0465-2_712. ISBN 978-1-4419-0426-3. S2CID 241324984.
- Darwin CR (1871). The Descent of Man and Selection in Relation to Sex. John Murray. ISBN 978-0-8014-2085-6.
- Curnoe D (June 2010). "A review of early Homo in southern Africa focusing on cranial, mandibular and dental remains, with the description of a new species (Homo gautengensis sp. nov.)". Homo. 61 (3): 151–177. doi:10.1016/j.jchb.2010.04.002. PMID 20466364.
- Sigmon 1981, p. 231.
- Sigmon 1981, p. 193.
- de Lumley MA (2015). "L'homme de Tautavel. Un Homo erectus européen évolué. Homo erectus tautavelensis" [Tautavel Man. An evolved European Homo erectus. Homo erectus tautavelensis]. L'Anthropologie (in French). 119 (3): 342–344. doi:10.1016/j.anthro.2015.06.001.
- Spoor F, Leakey MG, Gathogo PN, Brown FH, Antón SC, McDougall I, et al. (August 2007). "Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya". Nature. 448 (7154): 688–691. Bibcode:2007Natur.448..688S. doi:10.1038/nature05986. PMID 17687323. S2CID 35845.
- Zhu Z, Dennell R, Huang W, Wu Y, Qiu S, Yang S, et al. (July 2018). "Hominin occupation of the Chinese Loess Plateau since about 2.1 million years ago". Nature. 559 (7715): 608–612. Bibcode:2018Natur.559..608Z. doi:10.1038/s41586-018-0299-4. PMID 29995848. S2CID 49670311.
- Barras C (2018). "Tools from China are oldest hint of human lineage outside Africa". Nature. doi:10.1038/d41586-018-05696-8. ISSN 0028-0836. S2CID 188286436.
- Hao L, Chao Rong L, Kuman K (2017). "Longgudong, an Early Pleistocene site in Jianshi, South China, with stratigraphic association of human teeth and lithics". Science China Earth. 60 (3): 452–462. Bibcode:2017ScChD..60..452L. doi:10.1007/s11430-016-0181-1. S2CID 132479732.
- "Our direct human ancestor Homo erectus is older than we thought". EurekAlert. AAAS.
- Ferring R, Oms O, Agustí J, Berna F, Nioradze M, Shelia T, et al. (June 2011). "Earliest human occupations at Dmanisi (Georgian Caucasus) dated to 1.85-1.78 Ma". Proceedings of the National Academy of Sciences of the United States of America. 108 (26): 10432–10436. Bibcode:2011PNAS..10810432F. doi:10.1073/pnas.1106638108. PMC 3127884. PMID 21646521.
- Augusti J, Lordkipanidze D (June 2011). "How "African" was the early human dispersal out of Africa?". Quaternary Science Reviews. 30 (11–12): 1338–1342. Bibcode:2011QSRv...30.1338A. doi:10.1016/j.quascirev.2010.04.012.
- Rightmire GP (1998). "Human Evolution in the Middle Pleistocene: The Role of Homo heidelbergensis". Evolutionary Anthropology. 6 (6): 218–227. doi:10.1002/(sici)1520-6505(1998)6:6<218::aid-evan4>3.0.co;2-6. S2CID 26701026.
- Asfaw B, Gilbert WH, Beyene Y, Hart WK, Renne PR, WoldeGabriel G, et al. (March 2002). "Remains of Homo erectus from Bouri, Middle Awash, Ethiopia". Nature. 416 (6878): 317–320. Bibcode:2002Natur.416..317A. doi:10.1038/416317a. PMID 11907576. S2CID 4432263.
- Zaim Y, Ciochon RL, Polanski JM, Grine FE, Bettis EA, Rizal Y, et al. (October 2011). "New 1.5 million-year-old Homo erectus maxilla from Sangiran (Central Java, Indonesia)". Journal of Human Evolution. 61 (4): 363–376. doi:10.1016/j.jhevol.2011.04.009. PMID 21783226.
- Ciochon RL (June 2009). "The mystery ape of Pleistocene Asia". Nature. 459 (7249): 910–911. Bibcode:2009Natur.459..910C. doi:10.1038/459910a. PMID 19536242. S2CID 205047272.
- Kaifu Y, Baba H, Aziz F, Indriati E, Schrenk F, Jacob T (December 2005). "Taxonomic affinities and evolutionary history of the Early Pleistocene hominids of Java: dentognathic evidence". American Journal of Physical Anthropology. 128 (4): 709–726. doi:10.1002/ajpa.10425. PMID 15761880.
- Perkins S (2013). "Skull suggests three early human species were one". Nature. doi:10.1038/nature.2013.13972. S2CID 88314849.
- Lordkipanidze D, Ponce de León MS, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, Zollikofer CP (October 2013). "A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo". Science. 342 (6156): 326–331. Bibcode:2013Sci...342..326L. doi:10.1126/science.1238484. PMID 24136960. S2CID 20435482.
- Black R (17 October 2013). "Beautiful Skull Spurs Debate on Human History". National Geographic. Retrieved 6 June 2021.
- Sample I (17 October 2013). "Skull of Homo erectus throws story of human evolution into disarray". The Guardian.
- Giumares SW, Merino CL (September 2015). "Dmanisi hominin fossils and the problem of multiple species in the early Homo genus" (PDF). Nexus: The Canadian Student Journal of Anthropology. 23. S2CID 73528018. Archived from the original (PDF) on 14 January 2020.
- Argue D, Groves CP, Lee MS, Jungers WL (June 2017). "The affinities of Homo floresiensis based on phylogenetic analyses of cranial, dental, and postcranial characters". Journal of Human Evolution. 107: 107–133. doi:10.1016/j.jhevol.2017.02.006. PMID 28438318.
- Lordkipanidze D (4 October 2018). "Dmanisi". In Trevathan W, Cartmill M, Dufour D, Larsen C (eds.). The International Encyclopedia of Biological Anthropology. John Wiley & Sons, Inc. pp. 1–4. doi:10.1002/9781118584538.ieba0139. ISBN 9781118584422. S2CID 240090147.
- Baab K (December 2015). "Defining Homo erectus". Handbook of Paleoanthropology (2 ed.): 2189–2219. doi:10.1007/978-3-642-39979-4_73. ISBN 978-3-642-39978-7.
- Tattersall I, Schwartz J (2001). Extinct Humans. Boulder, Colorado: Westview/Perseus. ISBN 978-0-8133-3482-0.
- Kaifu Y, Aziz F, Indriati E, Jacob T, Kurniawan I, Baba H (October 2008). "Cranial morphology of Javanese Homo erectus: new evidence for continuous evolution, specialization, and terminal extinction". Journal of Human Evolution. 55 (4): 551–580. doi:10.1016/j.jhevol.2008.05.002. PMID 18635247.
- There was long-standing uncertainty whether H. floresiensis should be considered close to H. erectus, close to H. sapiens, or an altogether separate species. In 2017, it was suggested on morphological grounds that H. floresiensis is a sister species to either H. habilis or to a minimally habilis-erectus-ergaster-sapiens clade, and its line much more ancient than Homo erectus itself. Argue D, Groves CP, Lee MS, Jungers WL (June 2017). "The affinities of Homo floresiensis based on phylogenetic analyses of cranial, dental, and postcranial characters". Journal of Human Evolution. 107: 107–133. doi:10.1016/j.jhevol.2017.02.006. PMID 28438318.
- Kennedy KA, Sonakia A, Chiment J, Verma KK (December 1991). "Is the Narmada hominid an Indian Homo erectus?". American Journal of Physical Anthropology. 86 (4): 475–496. doi:10.1002/ajpa.1330860404. PMID 1776655.
- Krantz, G.S. (1975). "An explanation for the diastema of Javan erectus Skull IV". In: Paleoanthropology, Morphology and Paleoecology. La Hague: Mouton, 361–372.
- Zanolli C, Kullmer O, Kelley J, Bacon AM, Demeter F, Dumoncel J, et al. (May 2019). "Evidence for increased hominid diversity in the Early to Middle Pleistocene of Indonesia" (PDF). Nature Ecology & Evolution. 3 (5): 755–764. doi:10.1038/s41559-019-0860-z. PMID 30962558. S2CID 102353734.
- Baba H, Aziz F, Kaifu Y, Suwa G, Kono RT, Jacob T (February 2003). "Homo erectus calvarium from the Pleistocene of Java". Science. 299 (5611): 1384–1388. doi:10.1126/science.1081676. PMID 12610302. S2CID 20437090.
- Balzeau A (2006). "Are thickened cranial bones and equal participation of the three structural bone layers autapomorphic traits of Homo erectus?". Bulletins et mémoires de la Société d'Anthropologie de Paris. 18 (3–4): 145–163. doi:10.4000/bmsap.1528.
- Copes LE, Kimbel WH (January 2016). "Cranial vault thickness in primates: Homo erectus does not have uniquely thick vault bones". Journal of Human Evolution. 90: 120–134. doi:10.1016/j.jhevol.2015.08.008. PMID 26767964.
- Franciscus RG, Trinkaus E (April 1988). "Nasal morphology and the emergence of Homo erectus". American Journal of Physical Anthropology. 75 (4): 517–527. doi:10.1002/ajpa.1330750409. PMID 3133950.
- Jacobs LF (February 2019). "The navigational nose: a new hypothesis for the function of the human external pyramid". The Journal of Experimental Biology. 222 (Pt Suppl 1): jeb186924. doi:10.1242/jeb.186924. PMID 30728230.
- Antón SC, Taboada HG, Middleton ER, Rainwater CW, Taylor AB, Turner TR, et al. (July 2016). "Morphological variation in Homo erectus and the origins of developmental plasticity". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371 (1698): 20150236. doi:10.1098/rstb.2015.0236. PMC 4920293. PMID 27298467.
- Clark, Gary; Henneberg, Maciej (2022). "Interpopulational variation in human brain size: Implications for hominin cognitive phylogeny". Anthropological Review. 84 (4): 405–429. doi:10.2478/anre-2021-0029.
- Ungar PS, Grine FE (2006). "Diet in Early Homo: A Review of the Evidence and a New Model of Adaptive Versatility". Annual Review of Anthropology. 35: 208–228. doi:10.1146/annurev.anthro.35.081705.123153.
- Alan Walker, Richard Leakey (1993). The Nariokotome Homo erectus skeleton. Harvard University Press. p. 412. ISBN 9780674600751. Retrieved 2 October 2022.
- Migliano AB, Guillon M (2012). "The Effects of Mortality, Subsistence, and Ecology on Human Adult Height and Implications for Homo Evolution". Current Anthropology. 53 (S6): 359–368. doi:10.1086/667694. S2CID 84442763.
- Antón, Susan C.; Taboada, Hannah G.; Middleton, Emily R.; Rainwater, Christopher W.; Taylor, Andrea B.; Turner, Trudy R.; Turnquist, Jean E.; Weinstein, Karen J.; Williams, Scott A. (5 July 2016). "Morphological variation in Homo erectus and the origins of developmental plasticity". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1698): 20150236. doi:10.1098/rstb.2015.0236. ISSN 0962-8436. PMC 4920293. PMID 27298467.
- Antón, Susan C.; Potts, Richard; Aiello, Leslie C. (4 July 2014). "Evolution of early Homo: An integrated biological perspective". Science. 345 (6192): 1236828. doi:10.1126/science.1236828. ISSN 0036-8075. PMID 24994657. S2CID 30188239.
- Antón, Susan C.; Taboada, Hannah G.; Middleton, Emily R.; Rainwater, Christopher W.; Taylor, Andrea B.; Turner, Trudy R.; Turnquist, Jean E.; Weinstein, Karen J.; Williams, Scott A. (5 July 2016). "Morphological variation in Homo erectus and the origins of developmental plasticity". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1698): 20150236. doi:10.1098/rstb.2015.0236. ISSN 0962-8436. PMC 4920293. PMID 27298467.
- Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S (November 2008). "A female Homo erectus pelvis from Gona, Ethiopia". Science. 322 (5904): 1089–1092. Bibcode:2008Sci...322.1089S. doi:10.1126/science.1163592. PMID 19008443. S2CID 22191315.
- Leakey, Mary D (1979). Olduvai Gorge: my search for early man. London: Collins. ISBN 9780002116138. OCLC 647137093.
- Plavcan JM (2012). "Body Size, Size Variation, and Sexual Size Dimorphism in Early Homo". Current Anthropology. 53 (S6): 309–423. doi:10.1086/667605. S2CID 84095311.
- "Rightmire GP. The Evolution of Homo Erectus: Comparative Anatomical Studies of an Extinct Human Species. Cambridge University Press; 1990". search.library.ucr.edu. Retrieved 5 May 2022.
- Ruff C (March 2008). "Femoral/humeral strength in early African Homo erectus". Journal of Human Evolution. 54 (3): 383–390. doi:10.1016/j.jhevol.2007.09.001. PMID 17977577.
- Hatala KG, Roach NT, Ostrofsky KR, Wunderlich RE, Dingwall HL, Villmoare BA, et al. (July 2016). "Footprints reveal direct evidence of group behavior and locomotion in Homo erectus". Scientific Reports. 6 (28766): 28766. Bibcode:2016NatSR...628766H. doi:10.1038/srep28766. PMC 4941528. PMID 27403790.
- Roach, & Richmond. (2015). "Clavicle length, throwing performance and the reconstruction of the Homo erectus shoulder". Journal of Human Evolution, 80(C), 107–113.
- Haeusler M, Schiess R, Boeni T (November 2011). "New vertebral and rib material point to modern bauplan of the Nariokotome Homo erectus skeleton" (PDF). Journal of Human Evolution. 61 (5): 575–582. doi:10.1016/j.jhevol.2011.07.004. PMID 21868059.
- Rogers AR, Iltis D, Wooding S (2004). "Genetic Variation at the MC1R Locus and the Time since Loss of Human Body Hair". Current Anthropology. 45 (1): 105–108. doi:10.1086/381006. S2CID 224795768.
- Gilligan I (2010). "The Prehistoric Development of Clothing: Archaeological Implications of a Thermal Model". Journal of Archaeological Method and Theory. 15: 15–80. doi:10.1007/s10816-009-9076-x. S2CID 143004288.
- Dávid-Barrett T, Dunbar RI (May 2016). "Bipedality and hair loss in human evolution revisited: The impact of altitude and activity scheduling". Journal of Human Evolution. 94: 72–82. doi:10.1016/j.jhevol.2016.02.006. PMC 4874949. PMID 27178459.
- Jablonski NG (March 2012). "Human skin pigmentation as an example of adaptive evolution". Proceedings of the American Philosophical Society. 156 (1): 45–57. JSTOR 23558077. PMID 23035389.
- Kappelman J, Alçiçek MC, Kazanci N, Schultz M, Ozkul M, Sen S (January 2008). "First Homo erectus from Turkey and implications for migrations into temperate Eurasia". American Journal of Physical Anthropology. 135 (1): 110–116. doi:10.1002/ajpa.20739. PMID 18067194.
- Best A, Kamilar JM (April 2018). "The evolution of eccrine sweat glands in human and nonhuman primates". Journal of Human Evolution. 117: 33–43. doi:10.1016/j.jhevol.2017.12.003. PMID 29544622.
- Pagel M, Bodmer W (2004). "The Evolution of Human Hairlessness: Cultural Adaptations and the Ectoparasite Hypothesis". Evolutionary Theory and Processes: Modern Horizons. Springer, Dordrecht. pp. 329–335. doi:10.1007/978-94-017-0443-4_17. ISBN 978-94-017-0443-4.
- Gile J (2010). "Naked Love: The Evolution of Human Hairlessness". Biological Theory. 5 (4): 326–336. doi:10.1162/BIOT_a_00062. S2CID 84164968.
- Coqueugniot H, Hublin JJ, Veillon F, Houët F, Jacob T (September 2004). "Early brain growth in Homo erectus and implications for cognitive ability". Nature. 431 (7006): 299–302. Bibcode:2004Natur.431..299C. doi:10.1038/nature02852. PMID 15372030. S2CID 4428043.
- Caspari R, Lee SH (July 2004). "Older age becomes common late in human evolution". Proceedings of the National Academy of Sciences of the United States of America. 101 (30): 10895–10900. doi:10.1073/pnas.0402857101. PMC 503716. PMID 15252198.
- Steudel-Numbers KL (November 2006). "Energetics in Homo erectus and other early hominins: the consequences of increased lower-limb length". Journal of Human Evolution. 51 (5): 445–453. doi:10.1016/j.jhevol.2006.05.001. PMID 16780923.
- Ben-Dor M, Gopher A, Hershkovitz I, Barkai R (2011). "Man the fat hunter: the demise of Homo erectus and the emergence of a new hominin lineage in the Middle Pleistocene (ca. 400 kyr) Levant". PLOS ONE. 6 (12): e28689. Bibcode:2011PLoSO...628689B. doi:10.1371/journal.pone.0028689. PMC 3235142. PMID 22174868.
- Boaz N, Ciochon R (2004). "Headstrong Hominids". Natural History. 113 (1): 28–34.
- Kennedy GE (1985). "Bone thickness in Homo erectus". Journal of Human Evolution. 14 (8): 699–708. doi:10.1016/S0047-2484(85)80052-X.
- Russell MD, Brown T, Garn SM, Giris F, Turkel S, İşcan MY, et al. (1985). "The Supraorbital Torus: 'A Most Remarkable Peculiarity'". Current Anthropology. 26 (3): 337–350. doi:10.1086/203279. S2CID 146857927.
- Willems EP, van Schaik CP (August 2017). "The social organization of Homo ergaster: Inferences from anti-predator responses in extant primates". Journal of Human Evolution. 109: 11–21. doi:10.1016/j.jhevol.2017.05.003. PMID 28688456.
- Carotenuto F, Tsikaridze N, Rook L, Lordkipanidze D, Longo L, Condemi S, Raia P (June 2016). "Venturing out safely: The biogeography of Homo erectus dispersal out of Africa". Journal of Human Evolution. 95: 1–12. doi:10.1016/j.jhevol.2016.02.005. hdl:10356/82274. PMID 27260171.
- Plavcan JM (2012). "Implications of Male and Female Contributions to Sexual Size Dimorphism for Inferring Behavior in the Hominin Fossil Record". International Journal of Primatology. 33 (6): 1364–1381. doi:10.1007/s10764-012-9642-z. S2CID 17850676.
- Melamed Y, Kislev ME, Geffen E, Lev-Yadun S, Goren-Inbar N (December 2016). "The plant component of an Acheulian diet at Gesher Benot Ya'aqov, Israel". Proceedings of the National Academy of Sciences of the United States of America. 113 (51): 14674–14679. doi:10.1073/pnas.1607872113. PMC 5187744. PMID 27930293.
- Steele TE (June 2010). "A unique hominin menu dated to 1.95 million years ago". Proceedings of the National Academy of Sciences of the United States of America. 107 (24): 10771–10772. Bibcode:2010PNAS..10710771S. doi:10.1073/pnas.1005992107. PMC 2890732. PMID 20534542.
- Joordens JC, Wesselingh FP, de Vos J, Vonhof HB, Kroon D (December 2009). "Relevance of aquatic environments for hominins: a case study from Trinil (Java, Indonesia)". Journal of Human Evolution. 57 (6): 656–671. doi:10.1016/j.jhevol.2009.06.003. PMID 19683789.
- Gowlett JA (June 2016). "The discovery of fire by humans: a long and convoluted process". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371 (1696): 20150164. doi:10.1098/rstb.2015.0164. PMC 4874402. PMID 27216521.
- Gowlett JA, Wrangham RW (2013). "Earliest fire in Africa: Towards the convergence of archaeological evidence and the cooking hypothesis". Azania: Archaeological Research in Africa. 48 (1): 5–30. doi:10.1080/0067270X.2012.756754. S2CID 163033909.
- Rhodin A, Pritchard P, van Dijk PP, Saumure R, Buhlmann K, Iverson J, Mittermeier R, eds. (2015). Conservation Biology of Freshwater Turtles and Tortoises. Chelonian Research Monographs. Vol. 5 (First ed.). Chelonian Research Foundation. p. 15. doi:10.3854/crm.5.000e.fossil.checklist.v1.2015. ISBN 978-0-9653540-9-7.
- Beck RB, Black L, Krieger LS, Naylor PC, Shabaka DI (1999). World History: Patterns of Interaction. Evanston, IL: McDougal Littell. ISBN 978-0-395-87274-1.
- Richards MP (December 2002). "A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence". European Journal of Clinical Nutrition. 56 (12): 1270–1278. doi:10.1038/sj.ejcn.1601646. PMID 12494313.
- de la Torre I (July 2016). "The origins of the Acheulean: past and present perspectives on a major transition in human evolution". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371 (1698): 20150245. doi:10.1098/rstb.2015.0245. PMC 4920301. PMID 27298475.
- Lepre CJ, Roche H, Kent DV, Harmand S, Quinn RL, Brugal JP, et al. (August 2011). "An earlier origin for the Acheulian". Nature. 477 (7362): 82–85. Bibcode:2011Natur.477...82L. doi:10.1038/nature10372. PMID 21886161. S2CID 4419567.
- Nowell A, Chang ML (2009). "The Case Against Sexual Selection as an Explanation of Handaxe Morphology" (PDF). PaleoAnthropology: 77–88.
- Wynn T (1979). "The Intelligence of Later Acheulean Hominids". Man. 14 (3): 371–391. doi:10.2307/2801865. JSTOR 2801865.
- Choi K, Driwantoro D (2007). "Shell tool use by early members of Homo erectus in Sangiran, central Java, Indonesia: cut mark evidence". Journal of Archaeological Science. 34 (1): 48–58. doi:10.1016/j.jas.2006.03.013.
- Joordens JC, d'Errico F, Wesselingh FP, Munro S, de Vos J, Wallinga J, et al. (February 2015). "Homo erectus at Trinil on Java used shells for tool production and engraving". Nature. 518 (7538): 228–231. Bibcode:2015Natur.518..228J. doi:10.1038/nature13962. PMID 25470048. S2CID 4461751.
- Turner J (1996). History and Science of Knots. World Scientific. pp. 6–8. ISBN 9789810224691.
- Hlubik S, Berna F, Feibel C, Braun D (2017). "Researching the Nature of Fire at 1.5 Mya on the Site of FxJj20 AB, Koobi Fora, Kenya, Using High-Resolution Spatial Analysis and FTIR Spectrometry". Current Anthropology. 58: S243–S257. doi:10.1086/692530. S2CID 148948219.
- Roebroeks W, Villa P (March 2011). "On the earliest evidence for habitual use of fire in Europe". Proceedings of the National Academy of Sciences of the United States of America. 108 (13): 5209–5214. Bibcode:2011PNAS..108.5209R. doi:10.1073/pnas.1018116108. PMC 3069174. PMID 21402905.
- Beaumont PB (2011). "The Edge: More on Fire-Making by about 1.7 Million Years Ago at Wonderwerk Cave in South Africa". Current Anthropology. 52 (4): 585–595. doi:10.1086/660919. S2CID 144176681.
- Sandgathe D (2017). "Identifying and Describing Pattern and Process in the Evolution of Hominin Use of Fire". Current Anthropology. 58: S360–S370. doi:10.1086/691459. hdl:11858/00-001M-0000-002C-0141-3. S2CID 165025762.
- Antón SC (2003). "Natural history of Homo erectus". American Journal of Physical Anthropology. 122 (S37): 126–170. doi:10.1002/ajpa.10399. PMID 14666536.
- Zhong M, Shi C, Gao X, Wu X, Chen F, Zhang S, Zhang X, Olsen JW (2013). "On the possible use of fire by Homo erectus at Zhoukoudian, China". Chinese Science Bulletin. 59 (3): 335–343. doi:10.1007/s11434-013-0061-0. S2CID 93590269.
- Musée de Préhistoire Terra Amata. "Le site acheuléen de Terra Amata" [The Acheulean site of Terra Amata]. Musée de Préhistoire Terra Amata (in French). Retrieved 10 June 2022.
- Leakey MD (1971). Olduvai Gorge: Volume 3, Excavations in Beds I and II, 1960-1963. Cambridge University Press. p. 24. ISBN 9780521077231.
- Ingold T (2000). "Building, dwelling, living: how animals and people make themselves at home in the world". The Perception of the Environment: Essays on Livelihood, Dwelling and Skill. Psychology Press. p. 184. ISBN 9780415228329.
- Sklenář K (1987). "The Lower Paleolithic Dwelling Structure at Přezletice and its Significance". Anthropologie. 25 (2): 101–103. JSTOR 26294864.
- Ullman M, Hovers E, Goren-Inbar N, Frumkin A (2013). "Levantine cave dwellers: geographic and environmental aspects of early humans use of caves, case study from Wadi Amud, northern Israel". International Congress of Speleology. 1.
- Toups MA, Kitchen A, Light JE, Reed DL (January 2011). "Origin of clothing lice indicates early clothing use by anatomically modern humans in Africa". Molecular Biology and Evolution. 28 (1): 29–32. doi:10.1093/molbev/msq234. PMC 3002236. PMID 20823373.
- "New evidence in search for the mysterious Denisovans". ScienceDaily. 23 March 2021. Retrieved 30 March 2021.
- Bednarik RG (1999). "Pleistocene seafaring in the Mediterranean". Anthropologie. 37 (3): 275–282. JSTOR 26294895.
- Bednarik RG (1998). "An experiment in Pleistocene seafaring" (PDF). The International Journal of Nautical Archaeology. 27 (2): 139–149. doi:10.1111/j.1095-9270.1998.tb00797.x.
- Détroit F, Mijares AS, Corny J, Daver G, Zanolli C, Dizon E, et al. (April 2019). "A new species of Homo from the Late Pleistocene of the Philippines" (PDF). Nature. 568 (7751): 181–186. Bibcode:2019Natur.568..181D. doi:10.1038/s41586-019-1067-9. PMID 30971845. S2CID 106411053.
- Spikins P, Needham A, Wright B, Dytham C, Gatta M, Hitchens G (2019). "Living to fight another day: The ecological and evolutionary significance of Neanderthal healthcare". Quaternary Science Reviews. 217: 98–118. Bibcode:2019QSRv..217...98S. doi:10.1016/j.quascirev.2018.08.011.
- Lordkipanidze D, Vekua A, Ferring R, Rightmire GP, Agusti J, Kiladze G, et al. (April 2005). "Anthropology: the earliest toothless hominin skull". Nature. 434 (7034): 717–718. Bibcode:2005Natur.434..717L. doi:10.1038/434717b. PMID 15815618. S2CID 52800194.
- Haeusler M, Schiess R, Boeni T (February 2013). "Evidence for juvenile disc herniation in a homo erectus boy skeleton" (PDF). Spine. 38 (3): E123–E128. doi:10.1097/BRS.0b013e31827cd245. PMID 23154836. S2CID 11534863.
- Soriano M (January 1970). "The fluoric origin of the bone lesion in the Pithecanthropus erectus femur". American Journal of Physical Anthropology. 32 (1): 49–57. doi:10.1002/ajpa.1330320107. PMID 4984453.
- Henshilwood CS, d'Errico F, Watts I (July 2009). "Engraved ochres from the Middle Stone Age levels at Blombos Cave, South Africa". Journal of Human Evolution. 57 (1): 27–47. doi:10.1016/j.jhevol.2009.01.005. PMID 19487016.
- d'Errico F, Moreno RG, Rifkin RF (2012). "Technological, elemental and colorimetric analysis of an engraved ochre fragment from the Middle Stone Age levels of Klasies River Cave 1, South Africa". J. Archaeol. Sci. 39 (4): 942–952. doi:10.1016/j.jas.2011.10.032.
- Callaway E (2014). "Homo erectus made world's oldest doodle 500,000 years ago". Nature News. doi:10.1038/nature.2014.16477. S2CID 164153158.
- Dickson DB (1992). The Dawn of Belief: Religion in the Upper Paleolithic of Southwestern Europe. University of Arizona Press. pp. 40–46. ISBN 978-0-8165-1336-9.
- Morriss-Kay GM (February 2010). "The evolution of human artistic creativity". Journal of Anatomy. 216 (2): 158–176. doi:10.1111/j.1469-7580.2009.01160.x. PMC 2815939. PMID 19900185.
- d'Errico F, Nowell A (2000). "A New Look at the Berekhat Ram Figurine: Implications for the Origins of Symbolism". Cambridge Archaeological Journal. 10 (1): 123–167. doi:10.1017/S0959774300000056. S2CID 163138037.
- Watts I (2014). "The red thread: pigment use and the evolution of collective ritual". The Social Origins of Language. Oxford University Press. pp. 222–223. ISBN 978-0-19-966533-4.
- de Lumley H, Boone Y (1976). "Les structures d'habitat au Paléolithique moyen" [Housing structures from the lower Paleolithic]. In de Lumley H, Guilaine J (eds.). La Préhistoire française: Les civilisations paléolithiques et mésolithiques de la France [French prehistory: the Paleolithic and Mesolithic civilizations of France]. Éditions du Centre national de la recherche scientifique. ISBN 978-2-222-01968-8.
- Wreschner EE, Bolton R, Butzer KW, Delporte H, Häusler A, Heinrich A, et al. (1980). "Red Ochre and Human Evolution: A Case for Discussion" (PDF). Current Anthropology. 21 (5): 632–633. doi:10.1086/202541. JSTOR 2741829. S2CID 88099778.
- Weidenreich F (1935). "The Sinanthropus Population of Choukoutien (Locality 1) with a Preliminary Report on New Discoveries". Bulletin of the Geological Society of China. 14 (4): 427–468. doi:10.1111/j.1755-6724.1935.mp14004001.x.
- Binford LR, Ho CK (1985). "Taphonomy at a Distance: Zhoukoudian, 'The Cave Home of Beijing Man'?". Current Anthropology. 26 (4): 413–442. doi:10.1086/203303. JSTOR 2742759. S2CID 147164100.
- Kohn M, Mithen S (1999). "Handaxes: products of sexual selection?". Antiquity. 73 (281): 518–526. doi:10.1017/S0003598X00065078. S2CID 162903453.
- Latimer B, Ohman J (2001). "Axial dysplasia in Homo erectus". Journal of Human Evolution. 40.
- Meyer M, Lordkipanidze D, Vekua A (2006). Language and empathy in Homo erectus: Behaviors suggested by a modern spinal cord from Dmanisi, but not Nariokotome. Annual meeting of the Paleoanthroplogy Society. San Juan, Puerto Rico.
- Schiess R, Haeusler M (March 2013). "No skeletal dysplasia in the Nariokotome boy KNM-WT 15000 (Homo erectus)--a reassessment of congenital pathologies of the vertebral column". American Journal of Physical Anthropology. 150 (3): 365–374. doi:10.1002/ajpa.22211. PMID 23283736.
- Schiess R, Boeni T, Rühli F, Haeusler M (February 2014). "Revisiting scoliosis in the KNM-WT 15000 Homo erectus skeleton" (PDF). Journal of Human Evolution. 67 (48–59): 48–59. doi:10.1016/j.jhevol.2013.12.009. PMID 24491377.
- Luef EM (2018). "Tracing the human brain's classical language areas in extant and extinct hominids". The talking species: Perspectives on the evolutionary, neuronal and cultural foundations of language. Uni-Press Graz. ISBN 978-3-902666-52-9.
- Capasso L, Michetti E, D'Anastasio R (December 2008). "A Homo erectus hyoid bone: possible implications for the origin of the human capability for speech". Collegium Antropologicum. 32 (4): 1007–1011. PMID 19149203.
- Hillert DG (2015). "On the Evolving Biology of Language". Frontiers in Psychology. 6: 1796. doi:10.3389/fpsyg.2015.01796. PMC 4656830. PMID 26635694.
- Martínez I, Arsuaga JL, Quam R, Carretero JM, Gracia A, Rodríguez L (January 2008). "Human hyoid bones from the middle Pleistocene site of the Sima de los Huesos (Sierra de Atapuerca, Spain)" (PDF). Journal of Human Evolution. 54 (1): 118–124. doi:10.1016/j.jhevol.2007.07.006. PMID 17804038.
- Martínez I, Rosa M, Arsuaga JL, Jarabo P, Quam R, Lorenzo C, et al. (July 2004). "Auditory capacities in Middle Pleistocene humans from the Sierra de Atapuerca in Spain". Proceedings of the National Academy of Sciences of the United States of America. 101 (27): 9976–9981. Bibcode:2004PNAS..101.9976M. doi:10.1073/pnas.0403595101. PMC 454200. PMID 15213327.
- Padmanaban D (6 November 2020). "Climate Change May Have Been a Major Driver of Ancient Hominin Extinctions". SAPIENS. Retrieved 9 November 2020.
- "Climate change likely drove early human species to extinction, modeling study suggests". phys.org. Retrieved 9 November 2020.
- Raia P, Mondanaro A, Melchionna M, Di Febbraro M, Diniz-Filho JA, Rangel TF, et al. (23 October 2020). "Past Extinctions of Homo Species Coincided with Increased Vulnerability to Climatic Change". One Earth. 3 (4): 480–490. Bibcode:2020OEart...3..480R. doi:10.1016/j.oneear.2020.09.007. hdl:2158/1211341. ISSN 2590-3330.
- Zanolli, Clément, et al. "Inner Tooth Morphology of Homo Erectus from Zhoukoudian. New Evidence from an Old Collection Housed at Uppsala University, Sweden." Journal of Human Evolution, vol. 116, Mar. 2018, pp. 1–13.
- Copes, Lynn E., and William H. Kimbel. "Cranial Vault Thickness in Primates: Homo Erectus Does Not Have Uniquely Thick Vault Bones." Journal of Human Evolution, vol. 90, Jan. 2016, pp. 120–134.
- Delson E, Harvati K, Reddy D, Marcus LF, Mowbray K, Sawyer GJ, et al. (April 2001). "The Sambungmacan 3 Homo erectus calvaria: a comparative morphometric and morphological analysis". The Anatomical Record. 262 (4): 380–397. doi:10.1002/ar.1048. PMID 11275970. S2CID 25438682.
- Ciochon R, Long VT, Larick R, González L, Grün R, de Vos J, et al. (April 1996). "Dated co-occurrence of Homo erectus and Gigantopithecus from Tham Khuyen Cave, Vietnam". Proceedings of the National Academy of Sciences of the United States of America. 93 (7): 3016–3020. Bibcode:1996PNAS...93.3016C. doi:10.1073/pnas.93.7.3016. PMC 39753. PMID 8610161.
- Schuster AM (September–October 1998). "New Skull from Eritrea". Archaeology. Retrieved 3 October 2015.
- Herries AI, Martin JM, Leece AB, Adams JW, Boschian G, Joannes-Boyau R, et al. (April 2020). "Contemporaneity of Australopithecus, Paranthropus, and early Homo erectus in South Africa". Science. 368 (6486): eaaw7293. doi:10.1126/science.aaw7293. hdl:11568/1040368. PMID 32241925. S2CID 214763272.
- Ni, Xijun; Ji, Qiang; Wu, Wensheng; Shao, Qingfeng; Ji, Yannan; Zhang, Chi; Liang, Lei; Ge, Junyi; Guo, Zhen; Li, Jinhua; Li, Qiang; Grün, Rainer; Stringer, Chris (28 August 2021). "Massive cranium from Harbin in northeastern China establishes a new Middle Pleistocene human lineage". The Innovation. 2 (3): 100130. Bibcode:2021Innov...200130N. doi:10.1016/j.xinn.2021.100130. ISSN 2666-6758. PMC 8454562. PMID 34557770.
Further reading
- Leakey R, Walker A (November 1985). "Homo Erectus Unearthed". National Geographic. Vol. 168, no. 5. pp. 624–629. ISSN 0027-9358. OCLC 643483454.
- Sigmon BA, Cybulski JS (1981). Homo erectus: Papers in Honor of Davidson Black. University of Toronto Press. JSTOR 10.3138/j.ctvcj2jdw.11.
- Theunissen B, Theunissen LT (2012). Eugène Dubois and the Ape-Man from Java. Springer Netherlands. ISBN 9789400922099.
External links
- Homo erectus Origins – Exploring the Fossil Record – Bradshaw Foundation
- Archaeology Info
- Homo erectus – The Smithsonian Institution's Human Origins Program
- Possible co-existence with Homo Habilis – BBC News
- John Hawks's discussion of the Kocabas fossil
- Peter Brown's Australian and Asian Palaeoanthropology
- The Age of Homo erectus – Interactive Map of the Journey of Homo erectus out of Africa
- Human Timeline (Interactive) – Smithsonian, National Museum of Natural History (August 2016).