Concept
Version 7
Created by Boundless
Hydrolysis
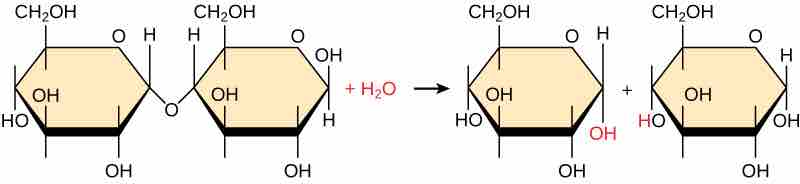
Hydrolysis reaction generating un-ionized products.
In the hydrolysis reaction shown here, the disaccharide maltose is broken down to form two glucose monomers with the addition of a water molecule. One glucose gets a hydroxyl group at the site of the former covalent bond, the other glucose gets a hydrogen atom. This is the reverse of the dehydration synthesis reaction joining these two monomers.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Synthesis of Biological Macromolecules. October 16, 2013."
http://cnx.org/content/m44397/latest/Figure_03_01_02.jpg
OpenStax CNX
CC BY 3.0.