Concept
Version 9
Created by Boundless
Electron Shells and the Bohr Model
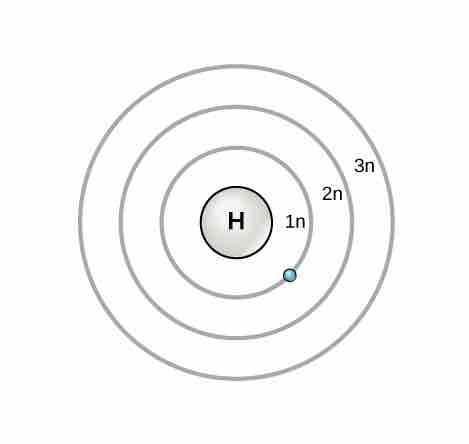
Orbitals in the Bohr model
The Bohr model was developed by Niels Bohr in 1913. In this model, electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. In the process, a photon of light is released.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013."
http://cnx.org/content/m44390/latest/Figure_02_01_05.png
OpenStax CNX
CC BY 3.0.