Concept
Version 7
Created by Boundless
Water’s Heat of Vaporization
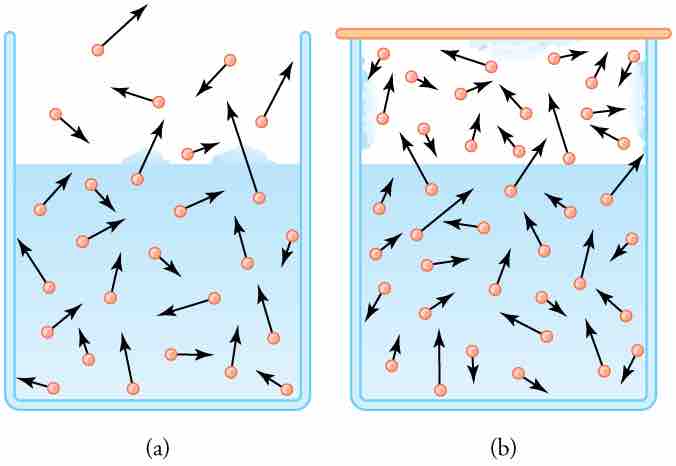
Humidity, Evaporation, and Boiling
(a) Because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. (b) If the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. This vapor density and the partial pressure it creates are the saturation values. They increase with temperature and are independent of the presence of other gases, such as air. They depend only on the vapor pressure of water.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Humidity, Evaporation, and Boiling. October 25, 2013."
http://cnx.org/content/m42219/1.5/
OpenStax CNX
CC BY 3.0.