The modern-day definition of a Lewis acid, as given by IUPAC, is a molecular entity—and corresponding chemical species—that is an electron-pair acceptor and therefore able to react with a Lewis base to form a Lewis adduct; this is accomplished by sharing the electron pair furnished by the Lewis base. Classically, the term "Lewis acid" was restricted to trigonal planar species with an empty p orbital, such as BR3 where R can be an organic substituent or a halide. However, metal ions such as Na+, Mg2+, and Ce3+ often form Lewis adducts upon reacting with a Lewis base.
Complex Ion Formation
Ligands create a complex when forming coordinate bonds with transition metals ions; the transition metal ion acts as a Lewis acid, and the ligand acts as a Lewis base. The number of coordinate bonds is known as the complex's coordination number. Common ligands include H2O and NH3 ; examples of complexes include the tetrachlorocobaltate(II) ion, [CoCl4]2- and the hexaqua-iron(III) ion, [Fe(H2O)6]3+.
Usually, metal complexes can only serve as Lewis acids after dissociating from a more weakly bound Lewis base, often water. For instance, Mg2+ can coordinate with ammonia in solutions, as shown below:
Nearly all compounds formed by the transition metals can be viewed as collections of the Lewis bases—or ligands—bound to the metal, which functions as the Lewis acid. The product is known as a complex ion, and the study of these ions is known as coordination chemistry. One coordination chemistry's applications is using Lewis bases to modify the activity and selectivity of metal catalysts in order to create useful metal-ligand complexes in biochemistry and medicine.
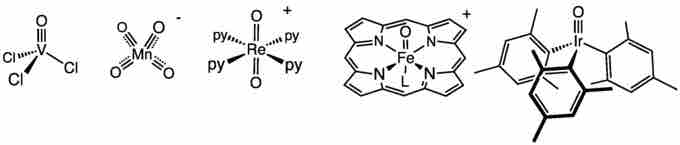
Examples of metal-ligand coordination complexes
Examples of several metals (V, Mn, Re, Fe, Ir) in coordination complexes with various ligands. All these metals act as Lewis acids, accepting electron pairs from their ligands.