Concept
Version 18
Created by Boundless
Introduction to Lewis Structures for Covalent Molecules
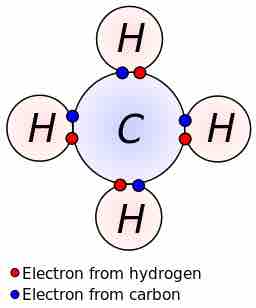
Lewis dot dragram for methane
Methane, with molecular formula CH4, is shown. The electrons are color-coded to indicate which atoms they belonged to before the covalent bonds formed, with red representing hydrogen and blue representing carbon. Four covalent bonds are formed so that C has an octet of valence electrons, and each H has two valence electrons—one from the carbon atom and one from one of the hydrogen atoms.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: