Introduction and History
A fuel cell is a device that converts the chemical energy from fuel into electricity via a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols are sometimes used. Fuel cells are different from batteries in that they require a constant source of fuel and oxygen to run, but they can produce electricity continually for as long as these inputs are supplied. The development of miniature fuel cells can provide a cheap, efficient, and reusable alternative to batteries.
William Grove developed the first crude fuel cells in 1839. The first commercial use of fuel cells was in NASA space programs to generate power for probes, satellites, and space capsules. Currently, fuel cells are used for primary and backup power for commercial, industrial, and residential buildings and in remote or inaccessible areas. They are used to power fuel-cell vehicles, including automobiles, buses, forklifts, airplanes, boats, motorcycles, and submarines.
Fuel Cell Structure and Function
There are many types of fuel cells, but they all consist of an anode, which is the negative side, a cathode, which is the positive side, and an electrolyte, which allows charges to move between the two sides of the fuel cell.

Fuel cell
Fuel cells convert the chemical energy from fuel into electricity via a chemical reaction with oxygen or another oxidizing agent. However, using hydrogen as the major fuel source in fuel cells has several pros and cons that have kept it controversial for mainstream use.
Electrons are drawn from the anode to the cathode through an external circuit, producing direct-current electricity. Fuel cells are classified by the electrolyte they use, which is the main difference among the various types of fuel cells. Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are "stacked," or placed in series, to increase the voltage. In addition to electricity, fuel cells produce water, heat, and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40-60 percent; it can reach 85 percent if waste heat is captured for use.
Despite the variety of fuel cell types, they all work in the same general manner. Two chemical reactions occur at the interfaces of the three different segments. The net result of the two reactions is that fuel is consumed, water or carbon dioxide is produced, and an electric current is created, which can be used to power electrical devices, normally referred to as the "load."
At the anode, a catalyst oxidizes the fuel, usually hydrogen, turning the fuel into a positively charged ion and a negatively charged electron. The electrolyte is a substance that is specifically designed so that ions can pass through it but electrons cannot. The freed electrons travel through a wire, creating the electric current. The ions travel through the electrolyte to the cathode. There, the ions are reunited with the electrons, and the two react with a third chemical, usually oxygen, to create water or carbon dioxide.
The Pros and Cons of Fuel Cells
The use of hydrogen fuel cells is controversial in some applications. First of all, since the energy used to produce the hydrogen is comparable to the energy in the hydrogen, it is inefficient, and therefore expensive. If conventional power plants were used to produce the hydrogen there would be, at best, no positive change in current pollution rates. Other types of fuel cells don't face this problem. For example, biological fuel cells take glucose and methanol from food scraps and convert them into hydrogen and food for the bacteria that break it down.
There are several advantages to hydrogen fuel cells, though. If the electricity produced by clean, renewable energy sources, such as solar and wind power, is used to produce hydrogen, the energy can be stored more easily than in large battery complexes.
There are practical problems to be overcome as well. Although the use of fuel cells for consumer products is probable in the near future, most current designs won't work if oriented upside-down. Additionally, current fuel cells cannot be scaled to the small size needed for portable devices such as cell phones. Current designs also require venting and therefore cannot operate under water. They may not be usable on aircraft because of the risk of fuel leaks through the vents. Finally, technologies for safe refueling of consumer fuel cells are not yet in place.
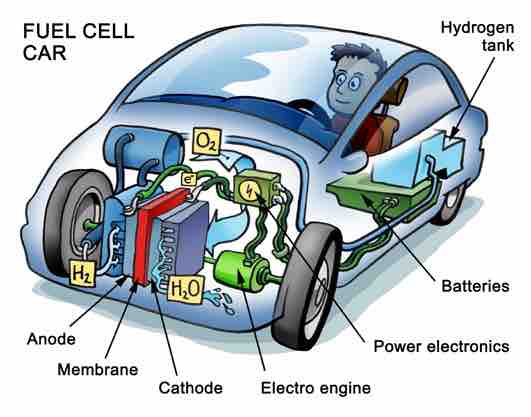
Fuel cell in a car
Fuel cells are a potential energy source for cars that do not run on gasoline. However, although fuel cells offer clean, renewable energy, there are several barriers to its widespread adoption.