Predicting the Redox Half-Reactions
Generally, the direction of a redox reaction depends on the relative strengths of oxidants and reductants in a solution. In simple situations, an electrochemical series can be very useful for determining the direction of the reaction.
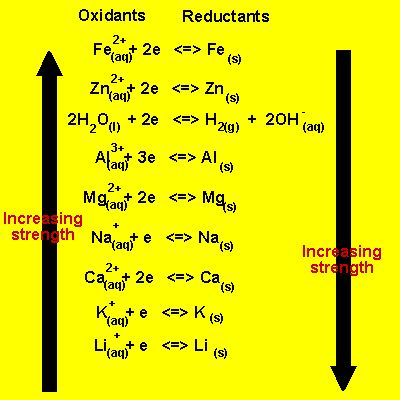
Electrochemical series
In order to predict if two reactants will take part in a spontaneous redox reaction, it is important to know how they rank in an electrochemical series.
In the table provided, the most easily reduced element is Li and the most easily oxidized is iron. This means that Li would be written as the reduction half-reaction when compared to any other element in this table. On the other hand, Fe would be written as the oxidation half-reaction when compared to any other element on this table.
Some reactions cannot be "eyeballed" in this manner. These reactions require a more mathematical method to determine the direction. To figure this out, it is important to consider the standard electrode potential, which is a measure of the driving force behind a reaction. The sign of the standard electrode potential indicates in which direction the reaction must proceed in order to achieve equilibrium.
For example, let's look at the reaction between zinc and acid:
Oxidation:
Reduction:
Eo = 0.76 V
The positive Eo value indicates that at STP this reaction must proceed to the right in order to achieve equilibrium. This is to say, a positive Eo value indicates a reaction has equilibrium constants that favor the products.
What happens to the standard electrode potential when the reaction is written in the reverse direction? Neither the relative strengths of the oxidizing or reducing agents nor the magnitude of the potential will change. However, what will change is the sign of the standard electrode potential. This means we can convert a spontaneous reaction to an unfavorable one and vice versa. For example if we turn the zinc oxidation half-reaction around (
The relative reactivities of different half-reactions can be compared to predict the direction of electron flow. Half-reaction equations can be combined if one is reversed to oxidation in a manner that cancels out the electrons.