Defining Electron Orbitals
The electron is a quantum particle and cannot have a distinct location, but the electron's orbital can be defined as the region of space around the nucleus in which the probability of finding the electron exceeds some arbitrary threshold value, such as 90% or 99%.
Because of the wave-like character of matter, the orbital corresponds to a standing-wave pattern in 3-dimensional space that we can often represent more clearly in a 2-dimensional cross section. The quantity that is varying ("waving") is a number denoted by ψ (psi), whose value varies from point to point according to the wavefunction for that particular orbital.
Orbitals of all types are simply mathematical functions that describe particular standing-wave patterns that can be plotted on a graph but have no physical reality of their own. Because of their wavelike nature, two or more orbitals (i.e., two or more functions ψ) can be combined both in-phase and out-of-phase to yield a pair of resultant orbitals that, to be useful, must have squares that describe actual electron distributions in the atom or molecule.
Molecular Orbitals and Their Phases
When combining orbitals to describe a bonding interaction between two species, the symmetry requirements for the system dictate that the two starting orbitals must make two new orbitals. One orbital, based on in-phase mixing of the orbitals, will be lower in energy and termed bonding. Another orbital, based on out-of-phase mixing of the orbitals, will be higher in energy and termed anti-bonding.
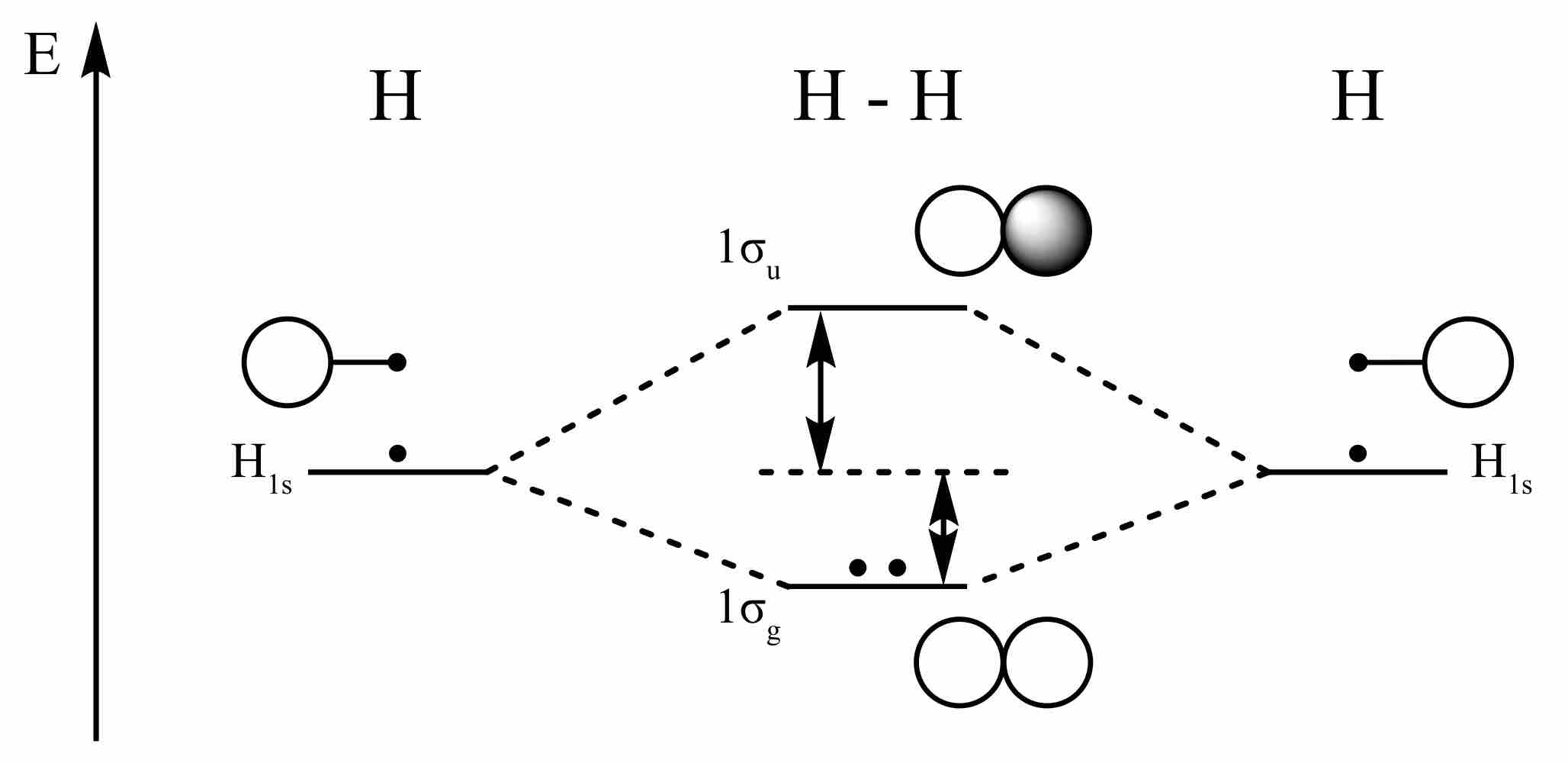
Hydrogen molecular orbitals
The dots here represent electrons. The in-phase combination of the s orbitals from the two hydrogen atoms provides a bonding orbital that is filled, whereas the out-of-phase combination provides an anti-bonding orbital that remains unfilled.
Orbitals that Overlap
Two atomic orbitals can overlap in two ways depending on their phase relationship. The phase of an orbital is a direct consequence of the wave-like properties of electrons. In graphical representations of orbitals, orbital phase is depicted either by a plus or minus sign (which have no relationship to electric charge) or by shading one lobe. The sign of the phase itself does not have physical meaning except when mixing orbitals to form molecular orbitals.
Constructive Overlap
Two same-sign orbitals have a constructive overlap forming a molecular orbital with the bulk of the electron density located between the two nuclei. This molecular orbital is called the bonding orbital and its energy is lower than that of the original atomic orbitals. A bond involving molecular orbitals that are symmetric with respect to rotation around the bond axis is called a sigma bond (σ-bond). If the phase changes, the bond becomes a pi bond (π-bond). Symmetry labels are further defined by whether the orbital maintains its original character after an inversion about its center; if it does, it is defined gerade (g), German for "straight." If the orbital does not maintain its original character, it is ungerade (u), German for "odd."
Destructive Overlap
Atomic orbitals can also interact with each other out-of-phase, which leads to destructive cancellation and no electron density between the two nuclei at the so-called nodal plane depicted as a perpendicular dashed line. In this anti-bonding molecular orbital with energy much higher than the original atomic orbitals, any electrons present are located in lobes pointing away from the central internuclear axis. For a corresponding σ-bonding orbital, such an orbital would be symmetrical but differentiated from it by an asterisk, as in σ*. For a π-bond, corresponding bonding and antibonding orbitals would not have such symmetry around the bond axis and would be designated π and π*, respectively.

Two p-orbitals forming a π-bond
If two parallel p-orbitals experience sideways overlap on adjacent atoms in a molecule, then a double or triple bond can develop. Although the π-bond is not as strong as the original σ-bond, its strength is added to the existing single bond.
P-orbital overlap is less than head-on overlap between two s orbitals in a σ-bond due to orbital orientation. This makes the π-bond a weaker bond than the original σ-bond that connects two neighboring atoms; however the fact that its strength is added to the underlying σ-bond bond makes for a stronger overall linkage. Electrons in π-bonds are often referred to as π- electrons. They limit rotational freedom about the double bond because a parallel orientation of the p-orbitals must be preserved to maintain the double or triple bond.