Chemical Properties of Oxides
An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2. Most of the Earth's crust consists of solid oxides, the result of elements being oxidized by the oxygen in air or water. Hydrocarbon combustion produces the two principal carbon oxides: carbon monoxide (CO) and carbon dioxide (CO2). Even materials considered pure elements often develop an oxide coating. For example, aluminum foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.
Oxygen Exhibits High Reactivity
Due to its electronegativity, oxygen forms stable chemical bonds with almost all elements to give the corresponding oxides. Noble metals (such as gold or platinum) are prized because they resist direct chemical combination with oxygen, and substances like gold (III) oxide must be generated by indirect routes. Two independent pathways for corrosion of elements are hydrolysis and oxidation by oxygen. The combination of water and oxygen is even more corrosive. Virtually all elements burn in an atmosphere of oxygen or an oxygen-rich environment. In the presence of water and oxygen (or simply air), some elements—for example, sodium—react rapidly, even dangerously, to give hydroxide products. In part for this reason, alkali and alkaline earth metals are not found in nature in their metallic form. Cesium is so reactive with oxygen that it is used as a getter in vacuum tubes. Solutions of potassium and sodium, are used to deoxygenate and dehydrate some organic solvents.
Passivation
The surface of most metals consists of oxides and hydroxides in the presence of air. As mentioned above, a well-known example is aluminum foil, which is coated with a thin film of aluminium oxide that passivates the metal, slowing further corrosion. The aluminium oxide layer can be built to greater thickness by the process of electrolytic anodising. Though solid magnesium and aluminium react slowly with oxygen at STP, they, like most metals, burn in air, generating very high temperatures.
Polymeric vs. Monomeric Molecular Structures
Oxides of most metals adopt polymeric structures with M-O-M crosslinks. Because these crosslinks are strong, the solids tend to be insoluble in solvents, though they are attacked by acids and bases. The formulas are often deceptively simple. Many are nonstoichiometric compounds. In these oxides, the coordination number of the oxide ligand is 2 for most electronegative elements, and 3–6 for most metals.
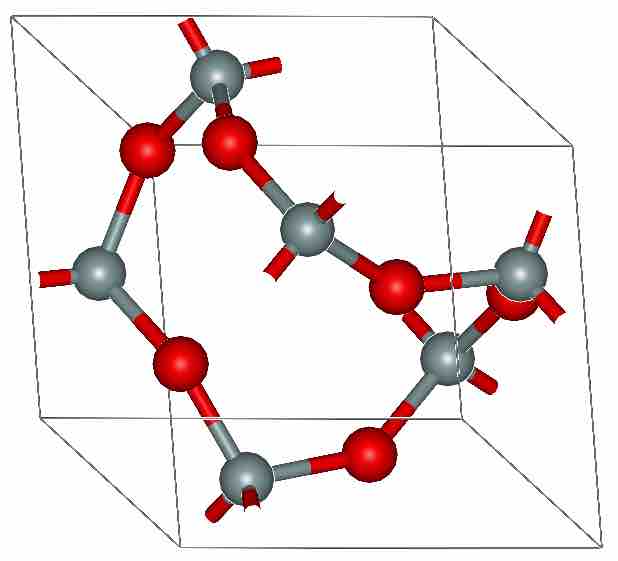
Silicon Dioxide
Silicon dioxide (SiO2) is one of the most common oxides on the surface of earth. Like most oxides, it adopts a polymeric structure.
Although most metal oxides are polymeric, some oxides are monomeric molecules. The most famous molecular oxides are carbon dioxide and carbon monoxide. Phosphorus pentoxide is a more complex molecular oxide with a deceptive name, the formula being P4O10. Some polymeric oxides (selenium dioxide and sulfur trioxide) depolymerize to give molecules when heated. Tetroxides are rare, and there are only five known examples: ruthenium tetroxide, osmium tetroxide, hassium tetroxide, iridium tetroxide, and xenon tetroxide. Many oxyanions are known, such as polyphosphates and polyoxometalates. Oxycations are rarer, an example being nitrosonium (NO+). Of course many compounds are known with both oxides and other groups. For the transition metals, many oxo-complexes are known, as well as oxyhalides.
Acid-Base Reactions
Oxides can be attacked by acids and bases. Those attacked only by acids are basic oxides; those attacked only by bases are acidic oxides. Oxides that react with both acids and bases are amphoteric. Metals tend to form basic oxides, non-metals tend to form acidic oxides, and amphoteric oxides are formed by elements near the boundary between metals and non-metals (metalloids).
Other Redox Reactions
Metals are "won" from their oxides by chemical reduction. A common and cheap reducing agent is carbon in the form of coke. The most prominent example is that of iron ore smelting.
Oxides, such as iron (III) oxide (or rust, which consists of hydrated iron (III) oxides Fe2O3·nH2O and iron (III) oxide-hydroxide FeO(OH), Fe(OH)3), form when oxygen combines with iron.
Metal oxides can be reduced by organic compounds. This redox process is the basis for many important transformations in chemistry, such as the detoxification of drugs by the P450 enzymes and the production of ethylene oxide, which is converted to antifreeze. In such systems the metal center transfers an oxide ligand to the organic compound, followed by the regeneration of the metal oxide, often by oxygen in air.