Aromatic Compounds
Aromatic compounds, originally named because of their fragrant properties, are unsaturated hydrocarbon ring structures that exhibit special properties, including unusual stability, due to their aromaticity. They are often represented as resonance structures containing single and double bonds. However, the bonding is stronger than expected for a conjugated structure, and it is more accurately depicted as delocalized electron density shared between all the atoms in the ring.
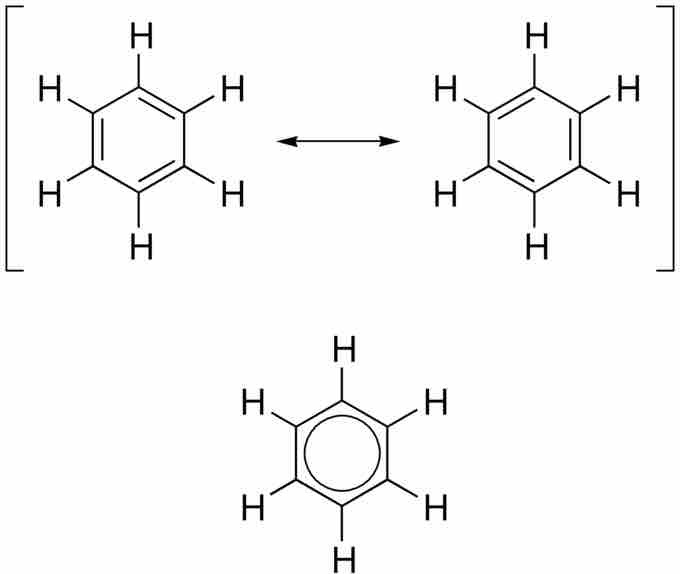
Benzene
Benzene can only be fully depicted with all of its resonance structures, which show how its pi-electrons are delocalized throughout its six-carbon ring. This delocalization leads to a lower overall energy for the molecule, giving it greater stability.
Structure of Aromatic Compounds
Aromatic compounds are cyclic structures in which each ring atom is a participant in a
Physical Properties of Aromatic Compounds
Aromatic compounds are generally nonpolar and immiscible with water. As they are often unreactive, they are useful as solvents for other nonpolar compounds. Due to their high ratio of carbon to hydrogen, aromatic compounds are characterized by a sooty yellow flame.
Reactivity of Aromatic Compounds
The double bonds in aromatic compounds are less likely to participate in addition reactions than those found in typical alkenes. Instead, cyclic aromatic compounds undergo electrophilic substitution reactions (reactions in which the ring acts as an nucleophile to a suitable electrophile). When benzene participates in such substitution reactions, the product retains the stability associated with the aromatic

Electrophilic Aromatic Substitution
The electron-rich benzene makes a bond with an electron-deficient chemical species (E+, the electrophile) which takes the place of an H-atom in the original structure. The reaction preserves the pi system of electrons and therefore the aromatic character of the benzene ring.
Sources of Aromatic Compounds
Aromatic compounds are produced from a variety of sources, including petroleum and coal tar. Poly-aromatic hydrocarbons are components of atmospheric pollution and are known carcinogens. Aromatic compounds are also interesting because of their presumed role in the origin of life as precursors to nucleotides and amino acids.