The electron affinity (Eea) of a neutral atom or molecule is defined as the amount of energy released when an electron is added to it to form a negative ion, as demonstrated by the following equation:
Electron affinity is measured for atoms and molecules in the gaseous state only, since in the solid or liquid states their energy levels would be changed by contact with other atoms or molecules. Robert S. Mulliken used a list of electron affinities to develop an electronegativity scale for atoms by finding the average of the electron affinity and ionization potential. A molecule or atom that has a more positive electron affinity value is often called an electron acceptor; one with a less positive electron affinity is called an electron donor. Together they may undergo charge-transfer reactions.
To use electron affinities properly, it is essential to keep track of the sign. For any reaction that releases energy, the change in energy (ΔE) has a negative value, and the reaction is called an exothermic process. Electron capture for almost all non-noble gas atoms involves the release of energy and therefore is an exothermic process.
Confusion may arise in mistaking Eea for ΔE. The numbers listed in tables of Eea are all positive because they are magnitudes; the values of Eea in a table of electron affinities all indicate the amount of energy released when an electron is added to an element. Because the release of energy is always an exothermic event, these all correspond to negative values of ΔE (indicating an exothermic process).
Periodic Trends in Electron Affinity
Although Eea varies greatly across the periodic table, some patterns emerge. Generally, nonmetals have more positive Eea than metals. Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher Eea. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values. Chlorine has the highest Eea while mercury has the lowest.
Eea generally increases across a period (row) in the periodic table, due to the filling of the valence shell of the atom. For instance, within the same period, a Group-17 atom releases more energy than a Group-1 atom upon gaining an electron because the added electron creates a filled valence shell and therefore is more stable.
A trend of decreasing Eea down the groups in the periodic table would be expected, since the additional electron is entering an orbital farther away from the nucleus. Since this electron is farther away, it should be less attracted to the nucleus and release less energy when added. However, this trend applies only to Group-1 atoms. Electron affinity follows the trend of electronegativity: fluorine (F) has a higher electron affinity than oxygen (O), and so on.
The trends noted here are very similar to those in ionization energy and change for similar (though opposing) reasons.
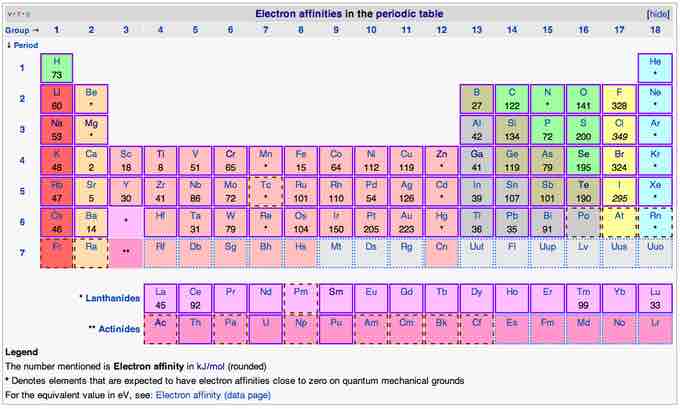
Electron affinities in the periodic table
This table shows the electron affinities in kJ/mol for the elements in the periodic table.