Cations and Anions form from Neutral Atoms
Every atom in its ground state is uncharged. It has, according to its atomic number, the same number of protons and electrons. Electrons are rather labile, however, and an atom will often gain or lose them depending on its electronegativity. The driving force for such gain or loss of electrons is the energetically optimal state of having a full valence (outermost) shell of electrons. In such a state, the resulting charged atom has the electron configuration of a noble gas.
Addition of an electron will disrupt the proton-electron balance and leave the atom negatively charged. Removal of an electron will, conversely, leave the atom positively charged. These charged atoms are known as ions.
Formation of Monatomic Ions
Monatomic ions are formed by the addition or removal of electrons from an atom's valence shell. The inner shells of an atom are filled with electrons that are tightly bound to the positively charged atomic nucleus and so do not participate in this kind of chemical interaction, but the valence shell can be very reactive depending on the atom and its electron configuration. The process of gaining or losing electrons from a neutral atom or molecule is called ionization.
Atoms can be ionized by bombardment with radiation, but the more purely chemical process of ionization is the transfer of electrons between atoms or molecules. This transfer is driven by the stabilization that comes by obtaining stable (full shell) electronic configurations. Atoms will gain or lose electrons depending on which action takes the least energy.
For example, Group 1 element sodium (Na) has a single electron in its valence shell, with full shells of 2 and 8 electrons beneath. Removal of this one electron leaves sodium stable: Its outermost shell now contains eight electrons, giving sodium the electron configuration of neon. Having gained a positive charge, the sodium ion is called a cation. The ionization of sodium can be chemically illustrated as follows:
Na → Na+ + e−
Sodium could gain electrons, but it would require seven more to achieve a full valence shell. Removing one electron is much easier than gaining seven, and thus sodium will in every chemical scenario achieve its octet by becoming a cation.
On the other hand, a chlorine atom (Cl) has seven electrons in its valence shell, which is one short of a stable, full shell with 8 electrons. Thus, a chlorine atom tends to gain an extra electron and attain a stable 8-electron configuration (the same as that of argon), becoming a negative chloride anion in the process:
Cl + e− → Cl−
Combining the propensity of sodium to lose an electron and of chloride to gain an electron, we observe complimentary reactivity. When combined, the uncharged atoms can exchange electrons and in doing so, achieve complete valence shells. The resulting ions stick together due to ionic bonds (opposite charges attract), leaving a crystal lattice structure of NaCl, more commonly known as rock salt. The reaction is as follows:
Na+ + Cl− → NaCl
Polyatomic and Molecular Ions
Ionization is not limited to individual atoms; polyatomic ions can also be formed. Polyatomic and molecular ions are often created by the addition or removal of elemental ions such as H+ in neutral molecules. For example, when ammonia, NH3, accepts a proton, H+, it forms the ammonium ion, NH4+. Ammonia and ammonium have the same number of electrons in essentially the same electronic configuration, but ammonium has an extra proton (the H+) that gives it a net positive charge.
Chemical Notation
When writing the chemical formula for an ion, its net charge is written in superscript immediately after the chemical structure for the molecule or atom. The net charge is written with the magnitude before the sign, that is, a doubly charged cation is indicated as 2+ instead of +2. However, the magnitude of the charge is omitted for singly charged molecules or atoms; for example, the sodium cation is indicated as Na+ and not Na1+.
An alternative way of showing a molecule or atom with multiple charges is by drawing out the signs multiple times; this is often seen with transition metals. Chemists sometimes circle the sign; this is merely ornamental and does not alter the chemical meaning. A twice-positively charged iron atom can also be expressed as Fe2+ or Fe++.
In the case of transition metals, oxidation states can be specified with Roman numerals; for example, Fe2+ is occasionally referred to as Fe(II) or FeII. The Roman numeral designates the formal oxidation state of an element, whereas the superscripted numerals denotes the net charge. The two notations are therefore exchangeable for monatomic ions, but the Roman numerals cannot be applied to polyatomic ions. However, it is possible to mix the notations for the individual metal center with a polyatomic complex, as demonstrated using the uranyl ion (UO2) as an example.
It should be noted that it is possible to remove many electrons from an atom. The energy required to do so may be recorded in a successive ionization energy diagram.
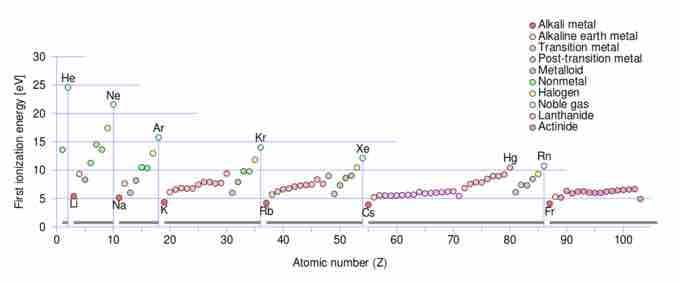
First ionization energy
Periodic trends for ionization energy (IE) vs. atomic number: note that within each of the seven periods the IE (colored circles) of an element begins at a minimum for the first column of the Periodic table (the alkali metals), and progresses to a maximum for the last column (the noble gases) which are indicated by vertical lines and labelled with a noble gas element symbol, and which also serve as lines dividing the 7 periods. Note that the maximum ionization energy for each row diminishes as one progresses from row 1 to row 7 in a given column, due to the increasing distance of the outer electron shell from the nucleus as inner shells are added.