Early philosophers and scientists appreciated that matter was composed of atoms and that many elements reacted in predictable proportions to each other. The periodic table was constructed in order to organize those observations and measurements. The principle of valence emerged, attributable to the presence or absence of electrons and the energy of those electrons in the volume around an atom's nucleus. Electrons, negatively charged subatomic particles, define an atom's chemical reactivity. Electron are organized in energy levels or electron shells, which correspond to the periods on the periodic table.
The Bohr Atom
Neils Bohr proposed a simplified picture of an atom, with a central nucleus surrounded by electrons in specific energy levels (n). The periodic table codifies the energy levels in periods, the rows on the table. The simplest atoms, hydrogen and helium, are found in row 1, or the first period. These atoms have electrons occupying the energy level n=1. Moving down, row 2, or period 2, contains the elements Li (lithium) through Ne (neon). The elements in period 2 have their level n=1 energy completely filled; they proceed to fill their n=2 level moving across the table to the right. In a similar fashion, moving down one period to row 3, there are the elements Na (sodium) through Ar (argon). The period-3 atoms have levels n=1 and n=2 filled; they are populating the n=3 level moving across the table.

A simplified depiction of atomic structure
With the nucleus at the center with a positive charge, the electrons are "organized" in energy levels, or shells, at increasing distances from the nucleus. The distance between the n = 2 and n = 3 shells in this illustration is the difference in energy between them.
It is important to remember that the periodic table is a representation of atoms with zero net charge; they have as many electrons around the nucleus as they have protons in the nucleus.
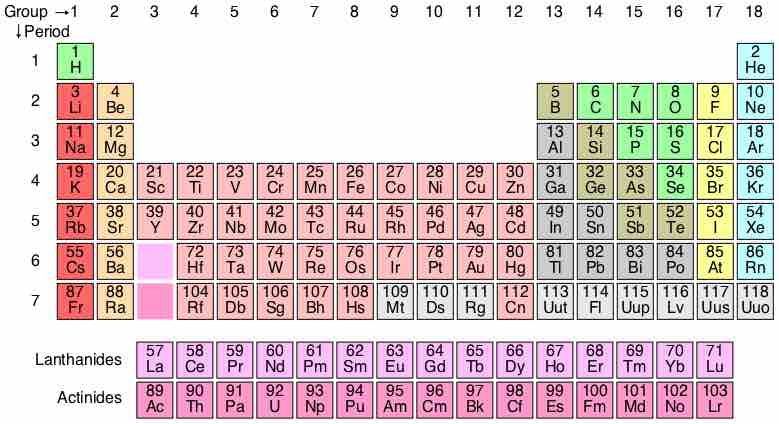
The periodic table
Elements are arranged according to electron configuration, with periodicities in valence.
The Aufbau Principle
In the n=1, n=2, and n=3 energy levels, electrons are organized in orbitals, designated as s, p, d, and f. For example, the atomic number of Ne (neon) is 10 and contains 5 orbitals (1s, 2s, 2px, 2py, and 2pz). In each full orbital, there are 2 electrons, giving a total of 10 to balance the positive charge provided by the 10 protons in the nucleus.

The filled orbitals of the neon atom
A depiction of the orbitals and their symmetries for the neon atom (Ne). The levels 1s and 2s are shown as spheroids, while the three 2p orbitals are shown as split spheroids. Each full orbital has 2 electrons, yielding 10 total for this element.
In the periodic table, there are 2 electrons in period 1, while both periods 2 and 3 have 8 electrons in the filled level. For atoms with atomic numbers less than about 20, the octet rule of electron addition and orbital filling applies. This simply states that the n=2 and n=3 levels, in particular, are full when there are 8 electrons. The Aufbau principal describes how electrons are put into orbitals in a particular order for filling.

The Aufbau principle
The Aufbau principle describes the incremental filling of orbitals and building atoms with known electronic configurations.