Valence Bond Theory
Valence bond theory is a synthesis of early understandings of how chemical bonds form. In 1916, G. N. Lewis proposed that the basis of chemical bonding is in the ability of atoms to share two bonding electrons. In 1927, physicist Walter Heitler and collaborator Fritz London developed the Heitler-London theory, which enabled the calculation of bonding properties of the covalently bonded diatomic hydrogen molecule (H2) based on quantum mechanical considerations. Finally, Linus Pauling integrated Lewis' proposal and the Heitler-London theory to give rise to two additional key concepts in valence bond theory: resonance and orbital hybridization.
According to Pauling's theory, a covalent bond is formed between two atoms by the overlap of their half-filled valence orbitals, each of which contains one unpaired electron. The bonds formed by participating atoms are considered to be weakly coupled overlapping orbitals. Valence bond structures are similar to Lewis structures, except where a single Lewis structure is insufficient, several valence bond structures can be used. It is in this aspect of valence bond theory that we see the concept of resonance.
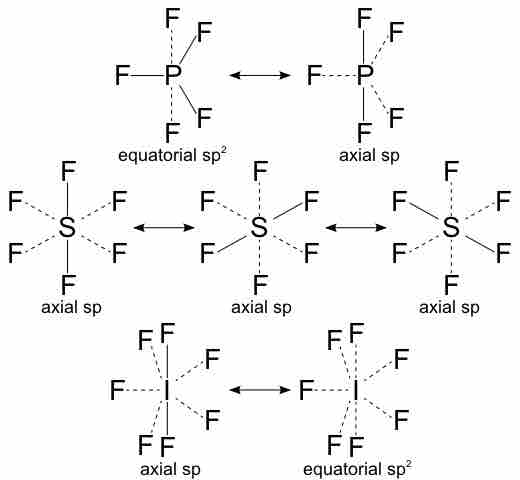
Valence structures of a hypervalent molecule
Several valence structures demonstrate the concept of resonance.
The two types of overlapping orbitals are sigma (σ) and pi (π) orbitals. Where bond order is concerned, single bonds are considered to be one sigma bond, double bonds are considered to contain one sigma and one pi bond, and triple bonds consist of one sigma bond and two pi bonds. Sigma bonds occur when the like orbitals of shared electrons overlap. For instance, when two s-orbital electrons overlap, we see a sigma bond. We see the concept of orbital hybridization arise when bonding orbitals share the characteristics of several types of orbitals.
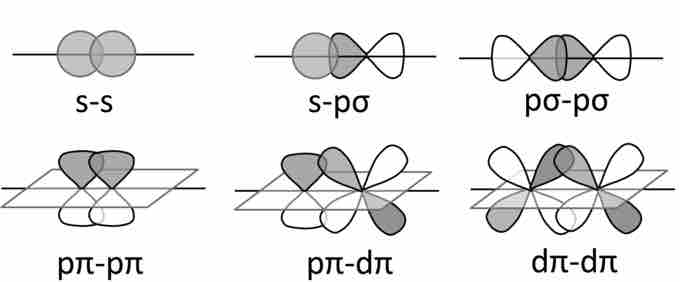
Orbital bonding
Sigma and pi bonding combinations of s-, p-, and d-orbitals.
When we apply valence bond theory to a coordination compound, the original electrons from the d orbital of the transition metal move into non-hybridized d orbitals. The electrons donated by the ligand move into hybridized orbitals of higher energy, which are then filled by electron pairs donated by the ligand.
Valence bond theory is used to explain covalent bond formation in many molecules, as it operates under the condition of maximum overlap, which leads to the formation of the strongest possible bonds.