Properties of Manganese
Manganese is a silvery-gray metal that resembles iron. It is hard and very brittle, difficult to fuse, but easy to oxidize. Manganese metal and its common ions are paramagnetic.
Oxidation States of Manganese
The most common oxidation states of manganese are 2+, 3+, 4+, 6+, and 7+. Mn2+ often competes with Mg2+ in biological systems. Manganese compounds where manganese is in oxidation state of 7+ are powerful oxidizing agents. Compounds with oxidation states 5+ (blue) and 6+ (green) are strong oxidizing agents.
Mn2+
The most stable oxidation state (oxidation number) for manganese is 2+, which has a pale pink color, and many manganese(II) compounds are common, such as manganese(II) sulfate (MnSO4) and manganese(II) chloride (MnCl2). The 2+ oxidation state is the state used in living organisms for essential functions; other states are toxic for the human body. The 2+ oxidation of manganese results from removal of the two 4s electrons, leaving a high spin ion in which all five of the 3d orbitals contain a single electron.
Mn3+
The 3+ oxidation state is seen in compounds like manganese(III) acetate; these are very powerful oxidizing agents. Solid compounds of manganese(III) are characterized by their preference for distorted octahedral coordination and their strong purple-red color.
Other Oxidation States of Manganese
The oxidation state 5+ can be obtained if manganese dioxide is dissolved in molten sodium nitrite. Manganate(VI) salts can also be produced by dissolving Mn compounds, such as manganese dioxide, in molten alkali while exposed to air. Permanganate (7+ oxidation state) compounds are purple and can give glass a violet color. Potassium permanganate, sodium permanganate, and barium permanganate are all potent oxidizers. Potassium permanganate finds use as a topical medicine (for example, in the treatment of fish diseases). Solutions of potassium permanganate were among the first stains and fixatives to be used in the preparation of biological cells and tissues for electron microscopy.
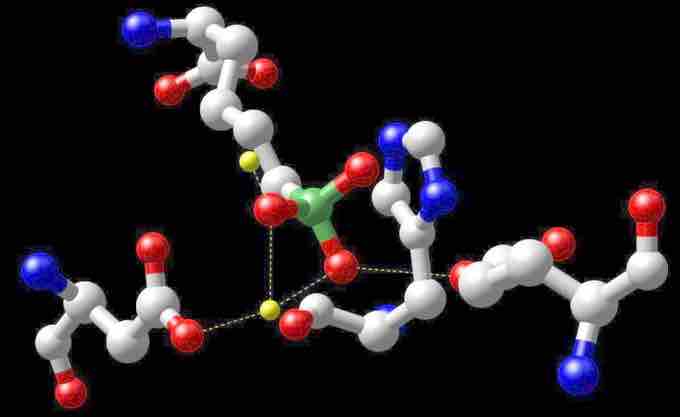
Arginase
Reactive center of arginase with boronic acid inhibitor - the manganese atoms are shown in yellow.
Manganese in Living Organisms
Manganese is an essential trace nutrient in all forms of life. The classes of enzymes that have manganese cofactors are very broad. The best-known manganese-containing polypeptides may be arginase, the diphtheria toxin, and Mn-containing superoxide dismutase (Mn-SOD). Mn-SOD is the type of SOD present in eukaryotic mitochondria and also in most bacteria (this fact is in keeping with the bacterial-origin theory of mitochondria). The Mn-SOD enzyme is probably one of the most ancient, as nearly all organisms living in the presence of oxygen use it to deal with the toxic effects of superoxide formed from the 1-electron reduction of dioxygen. The human body contains about 12 mg of manganese, which is stored mainly in the bones; in the tissue, it is mostly concentrated in the liver and kidneys. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes.