Section 4
Entropy
By Boundless
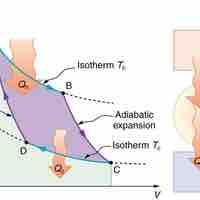
The entropy of a system is a measure of its disorder and of the unavailability of energy to do work.
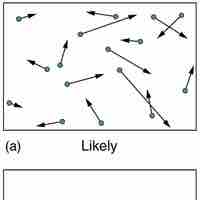
According the second law of thermodynamics, disorder is vastly more likely than order.
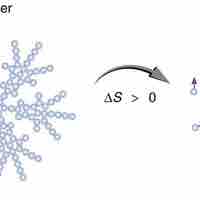
Entropy is a measure of disorder, so increased entropy means more disorder in the system.
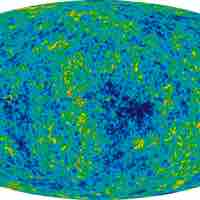
The entropy of the universe is constantly increasing and is destined for thermodynamic equilibrium, called the heat death of the universe.
It is possible for the entropy of one part of the universe to decrease, provided the total change in entropy of the universe increases.
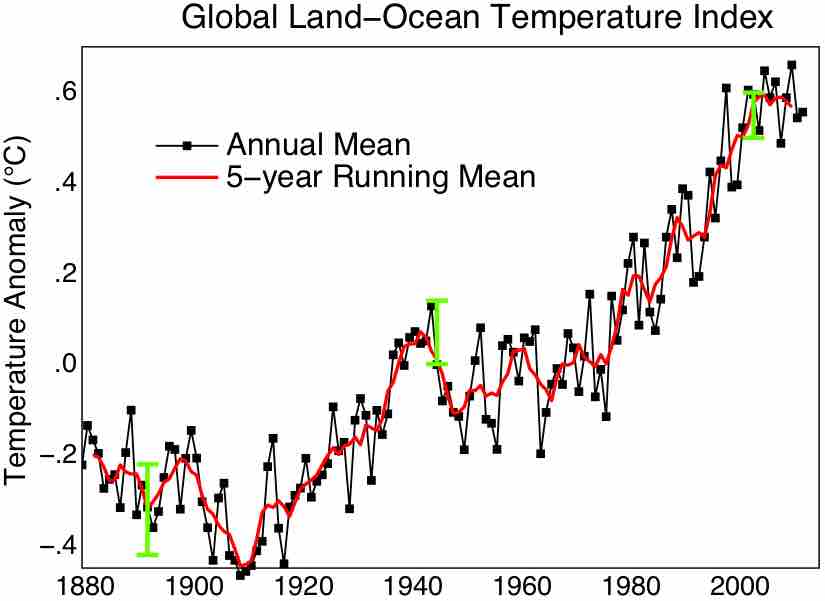
The Second Law of Thermodynamics may help provide explanation for the global warming over the last 250 years.

Thermal pollution is the degradation of water quality by any process that changes ambient water temperature.