In biochemistry, eicosanoids are signaling molecules made by oxidation of 20-carbon essential fatty acids (EFAs). The networks of controls that depend upon eicosanoids are among the most complex in the human body.
Eicosanoids are derived from either omega-3 (ω-3) or omega-6 (ω-6) EFAs. The ω-6 eicosanoids are generally pro-inflammatory; ω-3s are much less so. An excess of ω-6 to ω-3 fatty acids is common in western diets and is thought to encourage certain inflammatory disorders such as arthritis, cardiovascular disease, and cancers of the digestive system.
There are four families of eicosanoids:
- Prostaglandins
- Prostacyclins
- Thromboxanes
- Leukotrienes
For each, there are two or three separate series, derived either from an ω-3 or ω-6 EFA. These series' different activities largely explain the health effects of ω-3 and ω-6 fats.
Biosynthesis
Two families of enzymes catalyze EFA oxygenation to produce the eicosanoids:
- Cyclooxygenase, or COX, which generates the prostanoids.
- Lipoxygenase, or LOX, in several forms.
Eicosanoids are not stored within cells; they are synthesized as required. They derive from the EFAs that make up the cell and nuclear membranes. Biosynthesis is initiated when a cell is activated by mechanical trauma, cytokines, growth factors, or other stimuli.
Function and Pharmacology
Eicosanoids exert complex control over many bodily systems, mainly in inflammation or immunity, and as messengers in the central nervous system. Eicosanoids typically act as local hormones, acting on the same cell or nearby cells and then are rapidly inactivated.
Anti-inflammatory drugs such as aspirin and other NSAIDs act by down-regulating eicosanoid synthesis to prevent local and systemic inflammation.
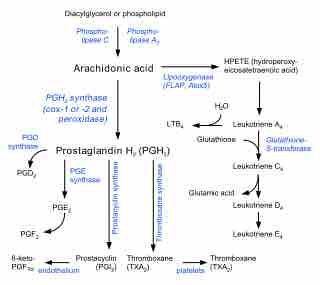
Biosynthesis of eicosanoids
Pathways in the biosynthesis of eicosanoids from arachidonic acid.