Dalton's law states that the total pressure exerted by the mixture of inert (non-reactive) gases is equal to the sum of the partial pressures of individual gases in a volume of air. This empirical law was observed by John Dalton in 1801 and is related to the ideal gas laws.
Dalton's Law in Respiration
The air in the atmosphere is a mixture of many different gases, that vary in concentration. Dalton's law states that at any given time, the percentage of each of these gasses in the air we breathe makes its contribution to total atmospheric pressure, and this contribution will depend on how much of each gas is in the air we breathe.
Dalton's law also implies that the relative concentration of gasses (their partial pressures) does not change as the pressure and volume of the gas mixture changes, so that air inhaled into the lungs will have the same relative concentration of gasses as atmospheric air. In the lungs, the relative concentration of gasses determines the rate at which each gas will diffuse across the alveolar membranes.
Mathematically, the pressure of a mixture of gases can be defined as the sum of the partial pressures of each of the gasses in air.
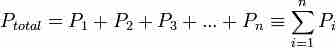
Dalton's law
In regards to atmospheric air, Dalton's law becomes:
For the purposes of gas exhange, O2 and CO2 are mainly considered due to their metabolic importance in gas exchange. Because gasses flow from areas of high pressure to areas of low pressure, atmospheric air has higher partial pressure of oxygen than alveolar air (PO2=159mmHg compared to PAO2=100 mmHg).
Similarly, atmospheric air has a much lower partial pressure for carbon dioxide compared to alveolar air (PCO2=.3mmHg compared to PACO2=40 mmHg). These pressure differences explain why oxygen flows into the alveoli and why carbon dioxide flows out of the alveoli through passive diffusion (just as a similar process explains alveolar and arterial gas exchange).
While inhaled air is similar to atmospheric air due to Dalton's law, exhaled air will have relative concentrations that are in between atmospheric and alveolar air due to the passive diffusion of gasses during gas exchange.
Dalton's law is only truly applicable in every situation to ideal gasses. Therefore most gasses will not follow it exactly, especially in conditions of extremely high pressure, or in situations where intermolecular forces act to keep the gasses together.