Electrochemical Gradients
Simple concentration gradients are differential concentrations of a substance across a space or a membrane, but in living systems, gradients are more complex. Because ions move into and out of cells and because cells contain proteins that do not move across the membrane and are mostly negatively charged, there is also an electrical gradient, a difference of charge, across the plasma membrane. The interior of living cells is electrically negative with respect to the extracellular fluid in which they are bathed. At the same time, cells have higher concentrations of potassium (K+) and lower concentrations of sodium (Na+) than does the extracellular fluid. In a living cell, the concentration gradient of Na+ tends to drive it into the cell, and the electrical gradient of Na+ (a positive ion) also tends to drive it inward to the negatively-charged interior. The situation is more complex, however, for other elements such as potassium. The electrical gradient of K+, a positive ion, also tends to drive it into the cell, but the concentration gradient of K+ tends to drive K+ out of the cell. The combined gradient of concentration and electrical charge that affects an ion is called its electrochemical gradient .
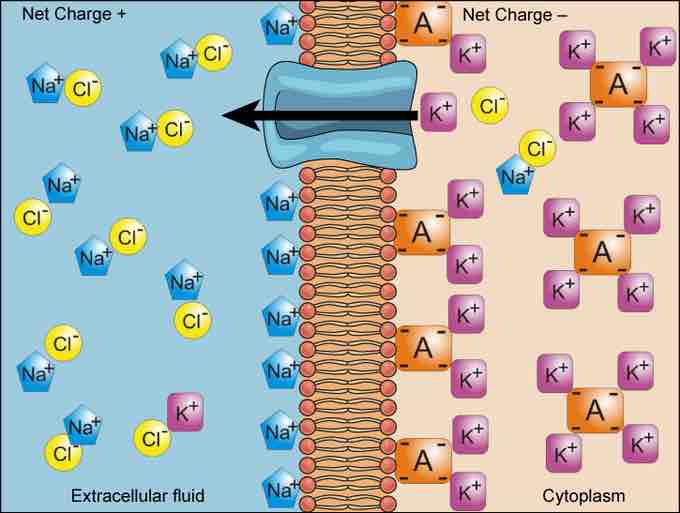
Electrochemical Gradient
Electrochemical gradients arise from the combined effects of concentration gradients and electrical gradients.
Moving Against a Gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from adenosine triphosphate (ATP) generated through the cell's metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the face of these passive movements. Much of a cell's supply of metabolic energy may be spent maintaining these processes. For example, most of a red blood cell's metabolic energy is used to maintain the imbalance between exterior and interior sodium and potassium levels required by the cell. Because active transport mechanisms depend on a cell's metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport describes the movement of material that is due to the electrochemical gradient established by primary active transport that does not directly require ATP.
Carrier Proteins for Active Transport
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement. There are three types of these proteins or transporters: uniporters, symporters, and antiporters . A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. These three types of carrier proteins are also found in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier protein pumps are Ca2+ ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively.
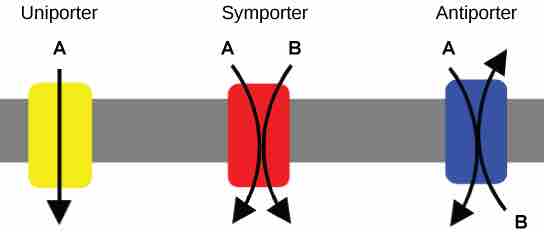
Uniporters, Symporters, and Antiporters
A uniporter carries one molecule or ion. A symporter carries two different molecules or ions, both in the same direction. An antiporter also carries two different molecules or ions, but in different directions.