The solubility product constant (Ksp) is the equilibrium constant for a solid that dissolves in an aqueous solution. All of the rules for determining equilibrium constants continue to apply. An equilibrium constant is the ratio of the concentration of the products of a reaction divided by the concentration of the reactants once the reaction has reached equilibrium. Consider this reaction:
The equilibrium expression for the reaction is:
Because the AgCl is a solid, its concentration before and after the reaction is the same. The equilibrium equation can therefore be rearranged as:
For substances in which the ions are not in a 1:1 ratio, the stoichiometric coefficients of the reaction become the exponents for the ions in the solubility-product expression:
Calculating the Solubility Product
At a certain temperature, the solubility of Fe(OH)2 in water is 7.7 x 10-6 mol/L (M).
Its Ksp can be calculated based on the equilibrium equation:
Therefore, the solubility product expression is:
One mole of dissolved Fe(OH)2 produces one mole of Fe2+ and two moles of OH-, so therefore:
2.jpg)
ICE table for the solubility of Fe(OH)2
The solubility of Fe(OH)2 is 7.7 x 10-6 M, this is equal to the value of the change (x) in the table.
Uses of Solubility Product
Solubility products are useful in predicting whether a precipitate will form under specified conditions. It is also helpful in choosing conditions under which two chemical substances in solution can be separated by fractional precipitation. The solubility product of a number of substances have been experimentally determined and can be used to predict solubility at a specified temperature.
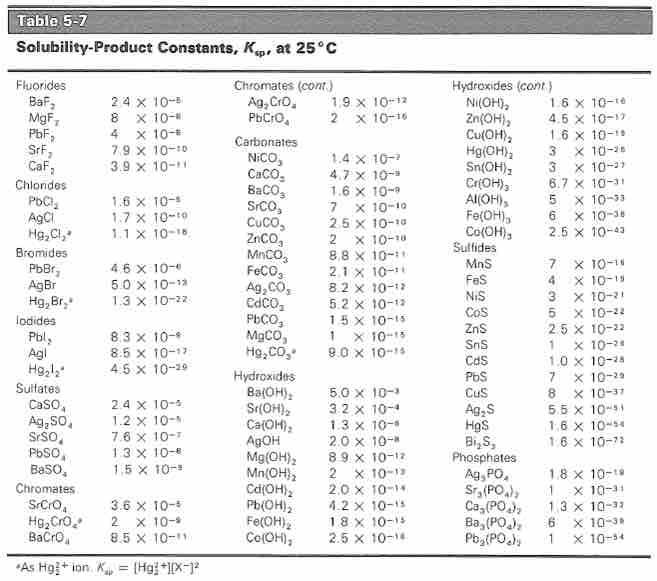
Solubility product constants of common ions
The solubility product constants of a number of substances. Substances are grouped by anion and listed in the order of decreasing Ksp; anions are listed roughly in order of decreasing solubility.