Definition of Strong Acids
The strength of an acid refers to the ease with which the acid loses a proton. A strong acid ionizes completely in an aqueous solution by losing one proton, according to the following equation:
where HA is a protonated acid, H+ is the free acidic proton, and A- is the conjugate base. Strong acids yield weak conjugate bases. For sulfuric acid, which is diprotic, the "strong acid" designation refers only to the dissociation of the first proton:
More precisely, the acid must be stronger in aqueous solution than a hydronium ion (H+), so strong acids have a pKa < -1.74. An example is hydrochloric acid (HCl), whose pKa is -6.3. This generally means that in aqueous solution at standard temperature and pressure, the concentration of hydronium ions is equal to the concentration of strong acid introduced to the solution.
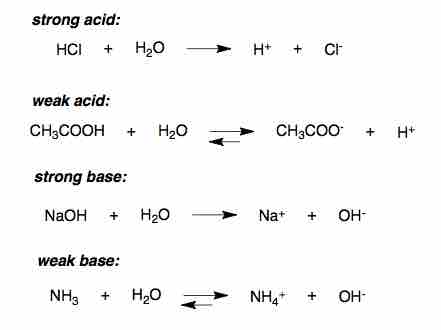
Ionization of acids and bases in water
A strong acid ionizes completely in an aqueous solution by losing one proton (H+).
Due to the complete dissociation of strong acids in aqueous solution, the concentration of hydronium ions in the water is equal to the total concentration (ionized and un-ionized) of the acid introduced to solution:
[H+] = [A−] = [HA]total and pH = −log[H+].
Strong acids, like strong bases, can cause chemical burns when exposed to living tissue.
Examples of Strong Acids
Some common strong acids (acids with pKa < -1) include:
- Hydroiodic acid (HI): pKa = -9.3
- Hydrobromic acid (HBr): pKa = -8.7
- Perchloric acid (HClO4): pKa ≈ -8
- Hydrochloric acid (HCl): pKa = -6.3
- Sulfuric acid (H2SO4): pKa1 ≈ -3 (first dissociation only)
- p-Toluenesulfonic acid: pKa = -2.8
- Nitric acid (HNO3): pKa ≈ -1.4
- Chloric acid (HClO3): pKa ≈ 1.0
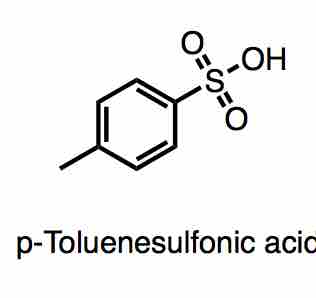
p-Toluenesolfonic acid
p-Toluenesulfonic acid is an example of an organic soluble strong acid, with a pKa of -2.8.
Strong Acid Catalysis
Strong acids can accelerate the rate of certain reactions. For instance, strong acids can accelerate the synthesis and hydrolysis of carbonyl compounds. With carbonyl compounds such as esters, synthesis and hydrolysis go through a tetrahedral transition state, where the central carbon has an oxygen, an alcohol group, and the original alkyl group. Strong acids protonate the carbonyl, which makes the oxygen positively charged so that it can easily receive the double-bond electrons when the alcohol attacks the carbonyl carbon; this enables ester synthesis and hydrolysis.